Effects of an Antioxidant-enriched Multivitamin in Cystic Fibrosis. A Randomized, Controlled, Multicenter Clinical Trial
- PMID: 29688760
- PMCID: PMC6118015
- DOI: 10.1164/rccm.201801-0105OC
Effects of an Antioxidant-enriched Multivitamin in Cystic Fibrosis. A Randomized, Controlled, Multicenter Clinical Trial
Abstract
Rationale: Cystic fibrosis (CF) is characterized by dietary antioxidant deficiencies, which may contribute to an oxidant-antioxidant imbalance and oxidative stress.
Objectives: Evaluate the effects of an oral antioxidant-enriched multivitamin supplement on antioxidant concentrations, markers of inflammation and oxidative stress, and clinical outcomes.
Methods: In this investigator-initiated, multicenter, randomized, double-blind, controlled trial, 73 pancreatic-insufficient subjects with CF 10 years of age and older with an FEV1 between 40% and 100% predicted were randomized to 16 weeks of an antioxidant-enriched multivitamin or control multivitamin without antioxidant enrichment. Endpoints included systemic antioxidant concentrations, markers of inflammation and oxidative stress, clinical outcomes (pulmonary exacerbations, anthropometric measures, pulmonary function), safety, and tolerability.
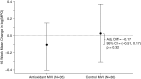
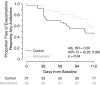
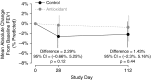
Measurements and main results: Change in sputum myeloperoxidase concentration over 16 weeks, the primary efficacy endpoint, was not significantly different between the treated and control groups. Systemic antioxidant (β-carotene, coenzyme Q10, γ-tocopherol, and lutein) concentrations significantly increased in the antioxidant-treated group (P < 0.001 for each), whereas circulating calprotectin and myeloperoxidase decreased in the treated group compared with the control group at Week 4. The treated group had a lower risk of first pulmonary exacerbation requiring antibiotics than the control group (adjusted hazard ratio, 0.50; P = 0.04). Lung function and growth endpoints did not differ between groups. Adverse events and tolerability were similar between groups.
Conclusions: Antioxidant supplementation was safe and well tolerated, resulting in increased systemic antioxidant concentrations and modest reductions in systemic inflammation after 4 weeks. Antioxidant treatment was also associated with a lower risk of first pulmonary exacerbation. Clinical trial registered with www.clinicaltrials.gov (NCT01859390).
Keywords: antioxidant; cystic fibrosis; inflammation; oxidative stress; pulmonary exacerbation.
Figures


Comment in
-
Low-Hanging Fruit and Antioxidant Therapy in Cystic Fibrosis.Am J Respir Crit Care Med. 2018 Sep 1;198(5):555-557. doi: 10.1164/rccm.201805-0872ED. Am J Respir Crit Care Med. 2018. PMID: 29847144 No abstract available.
References
-
- Nichols DP, Chmiel JF. Inflammation and its genesis in cystic fibrosis. Pediatr Pulmonol. 2015;50:S39–S56. - PubMed
-
- Homnick DN, Cox JH, DeLoof MJ, Ringer TV. Carotenoid levels in normal children and in children with cystic fibrosis. J Pediatr. 1993;122:703–707. - PubMed
-
- Kawchak DA, Sowell AL, Hofley PM, Zemel BS, Scanlin TF, Stallings VA. Longitudinal analysis shows serum carotenoid concentrations are low in children with cystic fibrosis. J Am Diet Assoc. 1999;99:1569–1572. - PubMed
-
- Feranchak AP, Sontag MK, Wagener JS, Hammond KB, Accurso FJ, Sokol RJ. Prospective, long-term study of fat-soluble vitamin status in children with cystic fibrosis identified by newborn screen. J Pediatr. 1999;135:601–610. - PubMed
-
- Wood LG, Fitzgerald DA, Gibson PG, Cooper DM, Collins CE, Garg ML. Oxidative stress in cystic fibrosis: dietary and metabolic factors. J Am Coll Nutr. 2001;20:157–165. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

