Impact evaluation of a community engagement intervention in improving childhood immunization coverage: a cluster randomized controlled trial in Assam, India
- PMID: 29688845
- PMCID: PMC5913885
- DOI: 10.1186/s12889-018-5458-x
Impact evaluation of a community engagement intervention in improving childhood immunization coverage: a cluster randomized controlled trial in Assam, India
Abstract
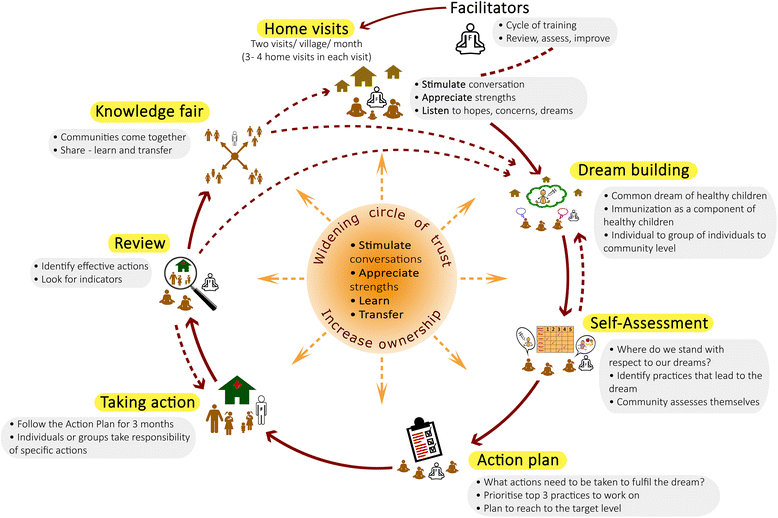
Background: To improve immunization coverage, most interventions that are part of the national immunization program in India address supply-side challenges. But, there is growing evidence that addressing demand-side factors can potentially contribute to improvement in childhood vaccination coverage in low- and middle-income countries. Participatory engagement of communities can address demand-side barriers while also mobilizing the community to advocate for better service delivery. The objective of this study is to evaluate the impact of a novel community engagement approach in improving immunization coverage. In our proposed intervention, we go a step beyond merely engaging the community and strive towards increasing 'ownership' by the communities.
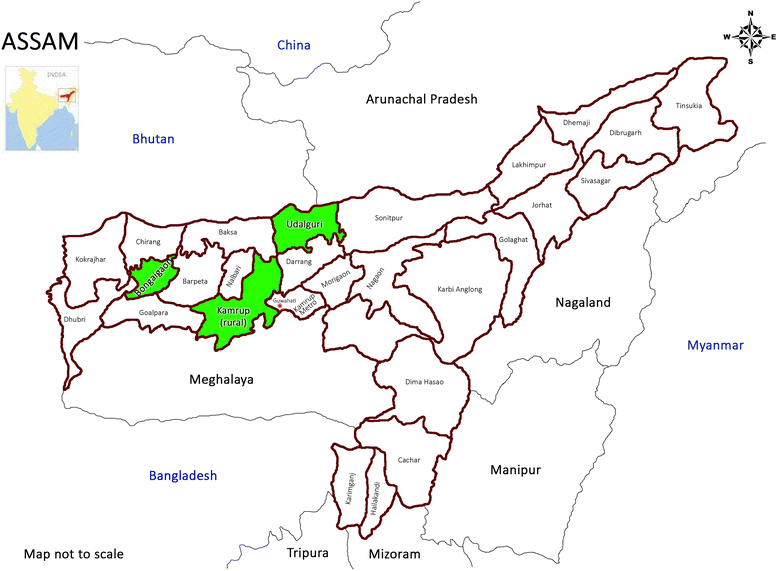
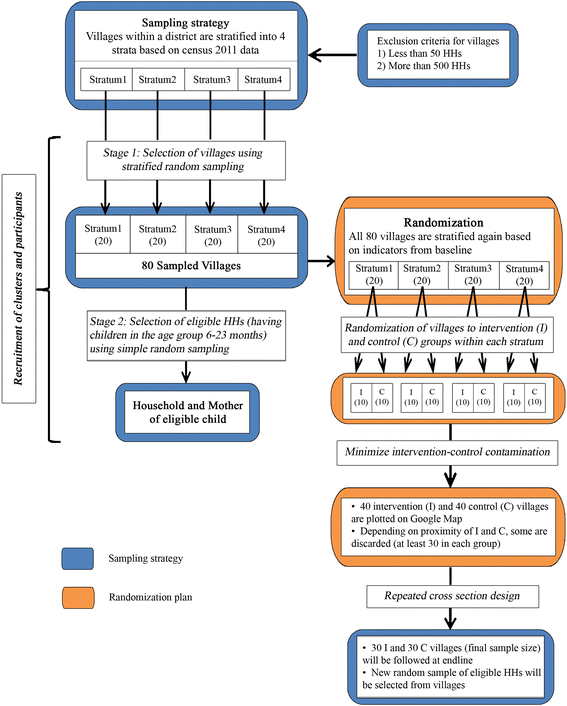
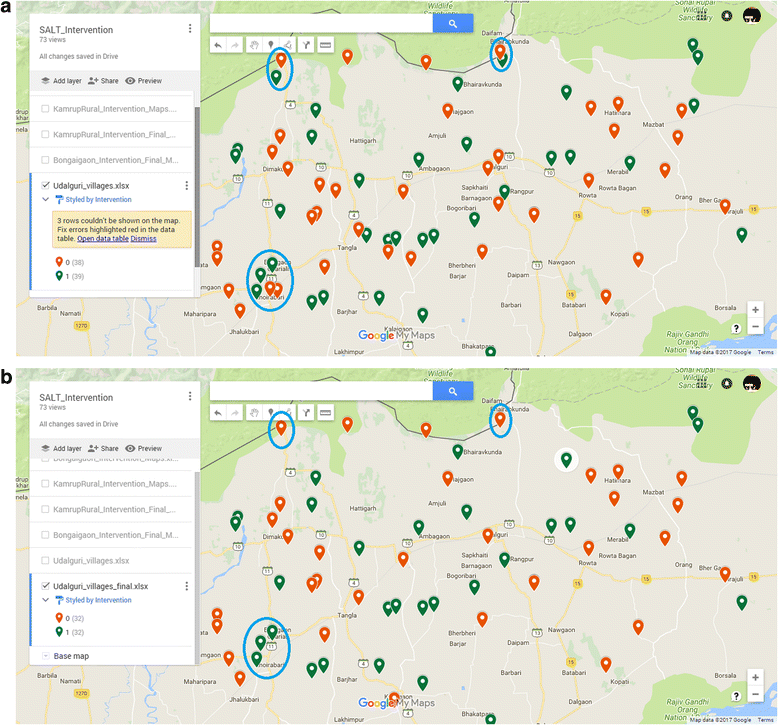
Methods/design: We adopt a cluster randomized design with two groups to evaluate the intervention in Assam, a state in the northeast region of India. To recruit villages and participants at baseline, we used a two-stage stratified random sampling method. We stratified villages; our unit of randomization, based on census data and randomly selected villages from each of the four strata. At the second-stage, we selected random sub-sample of eligible households (having children in the age group of 6-23 months) from each selected village. The study uses a repeated cross sectional design where we track the same sampled villages but draw independent random samples of households at baseline and endline. Total number of villages required for the study is 180 with 15 eligible HHs from each village. Post-baseline survey, we adopt a stratified randomization strategy to achieve better balance in intervention and control groups, leveraging information from the extensive baseline survey.
Discussion: The proposed intervention can help identify barriers to vaccination at the local level and potentially lead to more sustainable solutions over the long term. Our sampling design, sample size calculation, and randomization strategy address internal validity of our evaluation design. We believe that it would allow us to causally relate any observed changes in immunization coverage to the intervention.
Trial registration: The trial has been registered on 7th February, 2017 under the Clinical Trials Registry- India (CTRI), hosted at the ICMR's National Institute of Medical Statistics, having registration number CTRI/2017/02/007792 . This is the original study protocol.
Keywords: Community participation; DPT3; Demand-side intervention; Full immunization; North-East India; Ownership; Randomized evaluation; SALT; Study protocol; Universal immunization program.
Conflict of interest statement
Ethics approval and consent to participate
The IRB approval (TRC-IEC- 285/16) for the intervention and evaluation component of the study was received from the Institutional Ethics Committee (IEC) of Public Health Foundation of India (PHFI). Written informed consent was obtained from the participants. However, informed consent to randomization did not happen which does not seem to be a strict requirement for cluster randomized trials as the unit of randomization and the unit of observation are different [41].
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- WHO . Global Vaccine Action Plan 2011–2020. Geneva: World Health Organization; 2013.
-
- UNICEF India. Rapid Survey on Children (2013–14): National Report. New Delhi; 2015.
-
- UNICEF India. Coverage Evaluation Survey (CES), 2009: All India report. New Delhi; 2010.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

