Liver disease burden and required treatment expenditures for hepatitis C virus (HCV) infection in Thailand: Implications for HCV elimination in the new therapeutic era, a population-based study
- PMID: 29689073
- PMCID: PMC5916520
- DOI: 10.1371/journal.pone.0196301
Liver disease burden and required treatment expenditures for hepatitis C virus (HCV) infection in Thailand: Implications for HCV elimination in the new therapeutic era, a population-based study
Abstract
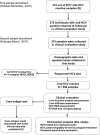
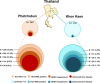
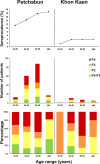
The prevalence of hepatitis C virus (HCV) infection has been decreasing globally, but the growing effects of HCV-related morbidity and mortality remain of concern. Advances in curative medicine, involving direct-acting antivirals (DAAs), have led many countries to aim to eradicate HCV. Information on epidemiology and disease burden is essential for national policy development. Thus, this study aimed to determine the HCV-related hepatic disease burden in areas of Thailand with high and average HCV prevalence in order to extrapolate the viral burden across Thailand. Patients previously diagnosed as positive for anti-HCV antibodies were recruited to assess chronic HCV infection (CHC) status, liver function, HCV-RNA level and hepatic fibrosis. The number of patients eligible for Universal Health Coverage (UC) scheme and the approximately required expenditure on interferon (IFN)-based treatment were estimated. In areas of both high (12%) and average (2%) HCV viremic prevalence, over half of the patients (52.2% to 62.5%) had advanced liver fibrosis (F3 and F4). A striking percentage of patients with F4 (38.9%) were found in the high-prevalence area, while comparable proportions of advanced liver fibrosis presented in the two areas and disease burden peaked at 50-59 years. Under the current UC program treatment scenario, 78-83% of CHC patients with stage F2-F4 fibrosis were eligible for treatment. The estimated expenditure required for overall CHC treatment across the whole country was 1,240 million USD at this current status, but the declining cost of generic DAA-based therapy may reduce the requirement to <90 million USD. This study provides information on the estimated number of CHC patients, liver disease burden and expenditure requirements for Thailand. To eliminate HCV by 2030, proactive government strategies raising public health to minimize transmission and emphasizing targeted screen-and-treatment programs, novel therapeutic guideline development for decentralizing treatment, and effective budget allocation are urgently needed.
Conflict of interest statement
Figures
References
-
- Polaris Observatory HCV Collaborators. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol Hepatol. 2017;2(3):161–76. doi: 10.1016/S2468-1253(16)30181-9 . - DOI - PubMed
-
- Global Burden of Disease Liver Cancer Collaboration. The burden of primary liver cancer and underlying etiologies from 1990 to 2015 at the global, regional, and national level: results from the global burden of disease study 2015. JAMA Oncol. 2017. doi: 10.1001/jamaoncol.2017.3055 . - DOI - PMC - PubMed
-
- Falade-Nwulia O, Suarez-Cuervo C, Nelson DR, Fried MW, Segal JB, Sulkowski MS. Oral direct-acting agent therapy for hepatitis C virus infection: a systematic review. Ann Intern Med. 2017;166(9):637–48. doi: 10.7326/M16-2575 - DOI - PMC - PubMed
-
- Miller MM. Sofosbuvir-velpatasvir: a single-tablet treatment for hepatitis C infection of all genotypes. Am J Health Syst Pharm. 2017;74(14):1045–52. doi: 10.2146/ajhp60632 - DOI - PubMed
-
- World Health Organization. Global hepatitis report 2017. http://apps.who.int/iris/bitstream/10665/255016/1/9789241565455-eng.pdf?...
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

