Higher expression of miR-133b is associated with better efficacy of erlotinib as the second or third line in non-small cell lung cancer patients
- PMID: 29689091
- PMCID: PMC5916492
- DOI: 10.1371/journal.pone.0196350
Higher expression of miR-133b is associated with better efficacy of erlotinib as the second or third line in non-small cell lung cancer patients
Abstract
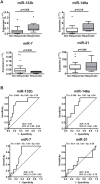
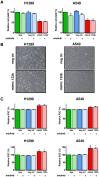
Epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (gefitinib, erlotinib and afatinib) are indicated as first-line therapy in patients with non-small cell lung cancer (NSCLC) whose tumors harbor activating mutations in the EGFR gene. Erlotinib is also used in second and third-line therapy for patients whose tumors have wild type EGFR but to date there are no validated biomarkers useful to identify which patients may benefit from this treatment. The expression level of four miRNAs: miR-133b, -146a, -7 and -21 which target EGFR was investigated by real-time PCR in tumor specimens from NSCLC patients treated with erlotinib administered as the second or third line. We found that miR-133b expression level better discriminated responder from non-responder patients to erlotinib. Higher levels of miR-133b in NSCLCs were associated with longer progression-free survival time of patients. Functional analyses on miR-133b through transfection of a miR-133b mimic in A549 and H1299 NSCLC cell lines indicated that increasing miR-133b expression level led to a decreased cell growth and altered morphology but did not affect sensitivity to erlotinib. The detection of miR-133b expression levels in tumors help in the identification of NSCLC patients with a better prognosis and who are likely to benefit from second and third-line therapy with erlotinib.
Conflict of interest statement
Figures

Similar articles
-
Tyr1068-phosphorylated epidermal growth factor receptor (EGFR) predicts cancer stem cell targeting by erlotinib in preclinical models of wild-type EGFR lung cancer.Cell Death Dis. 2015 Aug 6;6(8):e1850. doi: 10.1038/cddis.2015.217. Cell Death Dis. 2015. PMID: 26247735 Free PMC article.
-
Biomarkers of erlotinib response in non-small cell lung cancer tumors that do not harbor the more common epidermal growth factor receptor mutations.Int J Clin Exp Pathol. 2015 Mar 1;8(3):2888-98. eCollection 2015. Int J Clin Exp Pathol. 2015. PMID: 26045797 Free PMC article.
-
Exogenous Restoration of TUSC2 Expression Induces Responsiveness to Erlotinib in Wildtype Epidermal Growth Factor Receptor (EGFR) Lung Cancer Cells through Context Specific Pathways Resulting in Enhanced Therapeutic Efficacy.PLoS One. 2015 Jun 8;10(6):e0123967. doi: 10.1371/journal.pone.0123967. eCollection 2015. PLoS One. 2015. PMID: 26053020 Free PMC article.
-
Identifying activating mutations in the EGFR gene: prognostic and therapeutic implications in non-small cell lung cancer.J Bras Pneumol. 2015 Jul-Aug;41(4):365-75. doi: 10.1590/S1806-37132015000004531. J Bras Pneumol. 2015. PMID: 26398757 Free PMC article. Review.
-
MicroRNAs in non-small cell lung cancer: current status and future therapeutic promises.Curr Pharm Des. 2014;20(24):3982-90. doi: 10.2174/13816128113196660755. Curr Pharm Des. 2014. PMID: 24138721 Review.
Cited by
-
MicroRNA in lung cancer-a novel potential way for early diagnosis and therapy.J Appl Genet. 2023 Sep;64(3):459-477. doi: 10.1007/s13353-023-00750-2. Epub 2023 Feb 23. J Appl Genet. 2023. PMID: 36821071 Free PMC article. Review.
-
Dysregulated expression of microRNA involved in resistance to osimertinib in EGFR mutant non-small cell lung cancer cells.J Thorac Dis. 2023 Apr 28;15(4):1978-1993. doi: 10.21037/jtd-23-401. Epub 2023 Apr 24. J Thorac Dis. 2023. PMID: 37197540 Free PMC article.
-
microRNA-133b represses the progression of lung cancer through inhibiting SOX9/β-catenin signaling pathway.Int J Clin Exp Pathol. 2020 Sep 1;13(9):2270-2279. eCollection 2020. Int J Clin Exp Pathol. 2020. PMID: 33042331 Free PMC article.
-
MicroRNAs as the critical regulators of tyrosine kinase inhibitors resistance in lung tumor cells.Cell Commun Signal. 2022 Mar 9;20(1):27. doi: 10.1186/s12964-022-00840-4. Cell Commun Signal. 2022. PMID: 35264191 Free PMC article. Review.
-
miRNA Regulation of Glutathione Homeostasis in Cancer Initiation, Progression and Therapy Resistance.Microrna. 2020;9(3):187-197. doi: 10.2174/2211536609666191218103220. Microrna. 2020. PMID: 31849293 Free PMC article. Review.
References
-
- Travis WD, Brembilla E, Nicholson AG, Yatabe Y, Austin JH, Beasley MB, et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J Thorac Oncol. 2015;10(9):1243–60. doi: 10.1097/JTO.0000000000000630 - DOI - PubMed
-
- Sharma SV, Bell D, Settleman J, Haber DA. Epidermal growth factor receptor mutations in lung cancer. Nat Rev Cancer. 2007;7(3):169–81. doi: 10.1038/nrc2088 - DOI - PubMed
-
- Sequist LV, Joshi V, Jänne PA, Muzikansky A, Fidias P, Meyerson M, et al. Response to treatment and survival of patients with non-small cell lung cancer undergoing somatic EGFR mutation testing. Oncologist. 2007;12(1):90–8. - PubMed
-
- Muhsin M, Graham J, Kirkpatrick P. Gefitinib. Nat Rev Drug Discov. 2003;2(7):515–6. doi: 10.1038/nrd1136 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

