Characterization of Transglutaminase 2 activity inhibitors in monocytes in vitro and their effect in a mouse model for multiple sclerosis
- PMID: 29689097
- PMCID: PMC5918173
- DOI: 10.1371/journal.pone.0196433
Characterization of Transglutaminase 2 activity inhibitors in monocytes in vitro and their effect in a mouse model for multiple sclerosis
Erratum in
-
Correction: Characterization of Transglutaminase 2 activity inhibitors in monocytes in vitro and their effect in a mouse model for multiple sclerosis.PLoS One. 2018 Dec 13;13(12):e0209522. doi: 10.1371/journal.pone.0209522. eCollection 2018. PLoS One. 2018. PMID: 30543718 Free PMC article.
Abstract
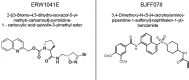
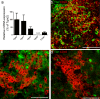
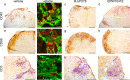
The neurodegenerative disease multiple sclerosis (MS) is pathologically characterized by the massive influx of immune cells into the central nervous system. This contributes to demyelination and axonal damage which causes symptoms such as motor and cognitive dysfunctions. The migration of leukocytes from the blood vessel is orchestrated by a multitude of factors whose determination is essential in reducing cellular influx in MS patients and the experimental autoimmune encephalomyelitis (EAE) animal model. The here studied enzyme tissue Transglutaminase (TG2) is present intracellularly, on the cell surface and extracellularly. There it contributes to cellular adhesion and migration via its transamidation activity and possibly by facilitating cellular interaction with the extracellular matrix. Previous data from our group showed reduced motor symptoms and cellular infiltration after using a pharmacological TG2 transamidation activity inhibitor in a rat EAE model. However, it remained elusive if the cross-linking activity of the enzyme resulted in the observed effects. To follow-up, we now characterized two new small molecule TG2 activity inhibitors, BJJF078 and ERW1041E. Both compounds are potent inhibitor of recombinant human and mouse Transglutaminase enzyme activity, mainly TG2 and the close related enzyme TG1. In addition they did not affect the binding of TG2 to the extracellular matrix substrate fibronectin, a process via which TG2 promotes cellular adhesion and migration. We found, that ERW1041E but not BJJF078 resulted in reduced EAE disease motor-symptoms while neither caused apparent changes in pathology (cellular influx), Transglutaminase activity or expression of inflammation related markers in the spinal cord, compared to vehicle treated controls. Although we cannot exclude issues on bioavailability and in vivo efficacy of the used compounds, we hypothesize that extracellular TG1/TG2 activity is of greater importance than (intra-)cellular activity in mouse EAE pathology.
Conflict of interest statement
Figures




Similar articles
-
Tissue Transglutaminase contributes to experimental multiple sclerosis pathogenesis and clinical outcome by promoting macrophage migration.Brain Behav Immun. 2015 Nov;50:141-154. doi: 10.1016/j.bbi.2015.06.023. Epub 2015 Jun 29. Brain Behav Immun. 2015. PMID: 26133787
-
Monocyte behaviour and tissue transglutaminase expression during experimental autoimmune encephalomyelitis in transgenic CX3CR1gfp/gfp mice.Amino Acids. 2017 Mar;49(3):643-658. doi: 10.1007/s00726-016-2359-0. Epub 2016 Nov 9. Amino Acids. 2017. PMID: 27826792 Free PMC article.
-
Tissue transglutaminase in marmoset experimental multiple sclerosis: discrepancy between white and grey matter.PLoS One. 2014 Jun 24;9(6):e100574. doi: 10.1371/journal.pone.0100574. eCollection 2014. PLoS One. 2014. PMID: 24959868 Free PMC article.
-
Recent Progress in the Development of Transglutaminase 2 (TGase2) Inhibitors.J Med Chem. 2017 Jan 26;60(2):554-567. doi: 10.1021/acs.jmedchem.6b01036. Epub 2016 Nov 21. J Med Chem. 2017. PMID: 28122456 Review.
-
Substrates, inhibitors, and probes of mammalian transglutaminase 2.Anal Biochem. 2020 Feb 15;591:113560. doi: 10.1016/j.ab.2019.113560. Epub 2019 Dec 24. Anal Biochem. 2020. PMID: 31874171 Free PMC article. Review.
Cited by
-
Tissue Transglutaminase Appears in Monocytes and Macrophages but Not in Lymphocytes in White Matter Multiple Sclerosis Lesions.J Neuropathol Exp Neurol. 2019 Jun 1;78(6):492-500. doi: 10.1093/jnen/nlz030. J Neuropathol Exp Neurol. 2019. PMID: 31058279 Free PMC article.
-
Neuronal and endothelial transglutaminase-2 expression in experimental autoimmune encephalomyelitis and multiple sclerosis.Neural Regen Res. 2022 Jul;17(7):1471-1472. doi: 10.4103/1673-5374.330598. Neural Regen Res. 2022. PMID: 34916421 Free PMC article. No abstract available.
-
Discovery and Characterization of PROTACs Targeting Tissue Transglutaminase (TG2).J Med Chem. 2023 Jul 27;66(14):9445-9465. doi: 10.1021/acs.jmedchem.2c01859. Epub 2023 Jul 14. J Med Chem. 2023. PMID: 37449845 Free PMC article.
-
Pathogenetic Contributions and Therapeutic Implications of Transglutaminase 2 in Neurodegenerative Diseases.Int J Mol Sci. 2024 Feb 17;25(4):2364. doi: 10.3390/ijms25042364. Int J Mol Sci. 2024. PMID: 38397040 Free PMC article. Review.
-
Rapid discovery of Transglutaminase 2 inhibitors for celiac disease with boosting ensemble machine learning.Comput Struct Biotechnol J. 2024 Oct 16;23:3669-3679. doi: 10.1016/j.csbj.2024.10.019. eCollection 2024 Dec. Comput Struct Biotechnol J. 2024. PMID: 39498152 Free PMC article.
References
-
- Compston A, Coles A. Multiple sclerosis. Lancet (London, England). 2008;372(9648):1502–17. - PubMed
-
- Bobholz JA, Rao SM. Cognitive dysfunction in multiple sclerosis: a review of recent developments. Current opinion in neurology. 2003;16(3):283–8. doi: 10.1097/01.wco.0000073928.19076.84 - DOI - PubMed
-
- Sospedra M, Martin R. Immunology of multiple sclerosis. Annu Rev Immunol. 2005;23:683–747. doi: 10.1146/annurev.immunol.23.021704.115707 - DOI - PubMed
-
- Kornek B, Lassmann H. Neuropathology of multiple sclerosis-new concepts. Brain research bulletin. 2003;61(3):321–6. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

