Glycan modifications to the gp120 immunogens used in the RV144 vaccine trial improve binding to broadly neutralizing antibodies
- PMID: 29689099
- PMCID: PMC5916523
- DOI: 10.1371/journal.pone.0196370
Glycan modifications to the gp120 immunogens used in the RV144 vaccine trial improve binding to broadly neutralizing antibodies
Abstract
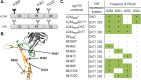
To date, the RV144 HIV vaccine trial has been the only study to show that immunization can confer protection from HIV infection. While encouraging, the modest 31.2% (P = 0.04) efficacy achieved in this study left significant room for improvement, and created an incentive to optimize the AIDSVAX B/E vaccine immunogens to increase the level of vaccine efficacy. Since the completion of the RV144 trial, our understanding of the antigenic structure of the HIV envelope protein, gp120, and of the specificity of broadly neutralizing monoclonal antibodies (bN-mAbs) that bind to it, has significantly improved. In particular, we have learned that multiple families of bN-mAbs require specific oligomannose glycans for binding. Both of the monomeric gp120 immunogens (MN- and A244-rgp120) in the AIDSVAX B/E vaccine used in the RV144 trial were enriched for glycans containing high levels of sialic acid, and lacked critical N-linked glycosylation sites required for binding by several families of bN-mAbs. The absence of these epitopes may have contributed to the low level of efficacy achieved in this study. In this report, we describe our efforts to improve the antigenic structure of the rgp120 immunogens used in the vaccine by optimizing glycan-dependent epitopes recognized by multiple bN-mAbs. Our results demonstrated that by shifting the location of one PNGS in A244-rgp120, and by adding two PNGS to MN-rgp120, in conjunction with the production of both proteins in a cell line that favors the incorporation of oligomannose glycans, we could significantly improve the binding by three major families of bN-mAbs. The immunogens described here represent a second generation of gp120-based vaccine immunogens that exhibit potential for use in RV144 follow-up studies.
Conflict of interest statement
Figures
Similar articles
-
Glycoform and net charge heterogeneity in gp120 immunogens used in HIV vaccine trials.PLoS One. 2012;7(8):e43903. doi: 10.1371/journal.pone.0043903. Epub 2012 Aug 22. PLoS One. 2012. PMID: 22928048 Free PMC article.
-
HIV-1 envelope proteins and V1/V2 domain scaffolds with mannose-5 to improve the magnitude and quality of protective antibody responses to HIV-1.J Biol Chem. 2014 Jul 25;289(30):20526-42. doi: 10.1074/jbc.M114.554089. J Biol Chem. 2014. PMID: 24872420 Free PMC article.
-
A Trimeric HIV-1 Envelope gp120 Immunogen Induces Potent and Broad Anti-V1V2 Loop Antibodies against HIV-1 in Rabbits and Rhesus Macaques.J Virol. 2018 Feb 12;92(5):e01796-17. doi: 10.1128/JVI.01796-17. Print 2018 Mar 1. J Virol. 2018. PMID: 29237847 Free PMC article.
-
The HIV-1 gp120 V1V2 loop: structure, function and importance for vaccine development.Expert Rev Vaccines. 2014 Dec;13(12):1489-500. doi: 10.1586/14760584.2014.951335. Epub 2014 Aug 28. Expert Rev Vaccines. 2014. PMID: 25163695 Review.
-
Progress in HIV vaccine development.Hum Vaccin Immunother. 2017 May 4;13(5):1018-1030. doi: 10.1080/21645515.2016.1276138. Epub 2017 Mar 10. Hum Vaccin Immunother. 2017. PMID: 28281871 Free PMC article. Review.
Cited by
-
Identification and CRISPR/Cas9 Inactivation of the C1s Protease Responsible for Proteolysis of Recombinant Proteins Produced in CHO Cells.Biotechnol Bioeng. 2019 Sep;116(9):2130-2145. doi: 10.1002/bit.27016. Epub 2019 Jun 10. Biotechnol Bioeng. 2019. PMID: 31087560 Free PMC article.
-
Glycosylation of viral surface proteins probed by mass spectrometry.Curr Opin Virol. 2019 Jun;36:56-66. doi: 10.1016/j.coviro.2019.05.003. Epub 2019 Jun 13. Curr Opin Virol. 2019. PMID: 31202133 Free PMC article. Review.
-
Glycosylation in health and disease.Nat Rev Nephrol. 2019 Jun;15(6):346-366. doi: 10.1038/s41581-019-0129-4. Nat Rev Nephrol. 2019. PMID: 30858582 Free PMC article. Review.
-
Production of a recombinant monoclonal antibody to Herpes Simplex Virus glycoprotein D for immunoaffinity purification of tagged proteins.J Immunol Methods. 2019 Feb;465:31-38. doi: 10.1016/j.jim.2018.11.015. Epub 2018 Nov 28. J Immunol Methods. 2019. PMID: 30502324 Free PMC article.
-
Gene editing in CHO cells to prevent proteolysis and enhance glycosylation: Production of HIV envelope proteins as vaccine immunogens.PLoS One. 2020 May 29;15(5):e0233866. doi: 10.1371/journal.pone.0233866. eCollection 2020. PLoS One. 2020. PMID: 32470085 Free PMC article.
References
-
- Haynes BF, Gilbert PB, McElrath MJ, Zolla-Pazner S, Tomaras GD, Alam SM, et al. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N Engl J Med. 2012;366(14):1275–86. Epub 2012/04/06. doi: 10.1056/NEJMoa1113425 ; PubMed Central PMCID: PMCPMC3371689. - DOI - PMC - PubMed
-
- de Bruyn G, Rossini AJ, Chiu YL, Holman D, Elizaga ML, Frey SE, et al. Safety profile of recombinant canarypox HIV vaccines. Vaccine. 2004;22(5–6):704–13. Epub 2004/01/27. . - PubMed
-
- Berman PW. Development of bivalent rgp120 vaccines to prevent HIV type 1 infection. AIDS Res Hum Retroviruses. 1998;14 Suppl 3:S277–89. Epub 1998/11/14. . - PubMed
-
- Berman PW, Huang W, Riddle L, Gray AM, Wrin T, Vennari J, et al. Development of bivalent (B/E) vaccines able to neutralize CCR5-dependent viruses from the United States and Thailand. Virology. 1999;265(1):1–9. Epub 1999/12/22. doi: 10.1006/viro.1999.0031 . - DOI - PubMed
-
- Pitisuttithum P, Berman PW, Phonrat B, Suntharasamai P, Raktham S, Srisuwanvilai LO, et al. Phase I/II study of a candidate vaccine designed against the B and E subtypes of HIV-1. J Acquir Immune Defic Syndr. 2004;37(1):1160–5. Epub 2004/08/21. . - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

