Ambient particulate matter activates the aryl hydrocarbon receptor in dendritic cells and enhances Th17 polarization
- PMID: 29689377
- PMCID: PMC5971007
- DOI: 10.1016/j.toxlet.2018.04.020
Ambient particulate matter activates the aryl hydrocarbon receptor in dendritic cells and enhances Th17 polarization
Abstract
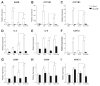
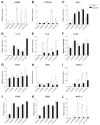
The objective of this study was to explore the role of the aryl hydrocarbon receptor (AhR) in ambient particulate matter (PM)-mediated activation of dendritic cells (DCs) and Th17-immune responses in vitro. To assess the potential role of the AhR in PM-mediated activation of DCs, co-stimulation, and cytokine expression, bone marrow (BM)-derived macrophages and DCs from C57BL/6 wildtype or AhR knockout (AhR-/-) mice were treated with PM. Th17 differentiation was assessed via co-cultures of wildtype or AhR-/- BMDCs with autologous naive T cells. PM2.5 significantly induced AhR DNA binding activity to dioxin responsive elements (DRE) and expression of the AhR repressor (AhRR), cytochrome P450 (CYP) 1A1, and CYP1B1, indicating activation of the AhR. In activated (OVA sensitized) BMDCs, PM2.5 induced interleukin (IL)-1β, CD80, CD86, and MHC class II, suggesting enhanced DC activation, co-stimulation, and antigen presentation; responses that were abolished in AhR deficient DCs. DC-T cell co-cultures treated with PM and lipopolysaccharide (LPS) led to elevated IL-17A and IL-22 expression at the mRNA level, which is mediated by the AhR. PM-treated DCs were essential in endowing T cells with a Th17-phenotype, which was associated with enhanced expression of MHC class II and cyclooxygenase (COX)-2. In conclusion, PM enhances DC activation that primes naive T cell differentiation towards a Th17-like phenotype in an AhR-dependent manner.
Keywords: Aryl hydrocarbon receptor (AhR); Dendritic cells (DCs); Particulate matter (PM); Polycyclic aromatic hydrocarbons (PAHs); Th17 cells.
Copyright © 2018 Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors do not have competing interests.
Figures


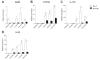
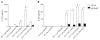

Similar articles
-
Polycyclic aromatic hydrocarbons (PAHs) present in ambient urban dust drive proinflammatory T cell and dendritic cell responses via the aryl hydrocarbon receptor (AHR) in vitro.PLoS One. 2018 Dec 21;13(12):e0209690. doi: 10.1371/journal.pone.0209690. eCollection 2018. PLoS One. 2018. PMID: 30576387 Free PMC article.
-
Radical containing combustion derived particulate matter enhance pulmonary Th17 inflammation via the aryl hydrocarbon receptor.Part Fibre Toxicol. 2018 May 3;15(1):20. doi: 10.1186/s12989-018-0255-3. Part Fibre Toxicol. 2018. PMID: 29724254 Free PMC article.
-
Association between particulate matter containing EPFRs and neutrophilic asthma through AhR and Th17.Respir Res. 2021 Oct 26;22(1):275. doi: 10.1186/s12931-021-01867-w. Respir Res. 2021. PMID: 34702270 Free PMC article.
-
Induction of CYP1A1. The AhR/DRE paradigm: transcription, receptor regulation, and expanding biological roles.Curr Drug Metab. 2001 Jun;2(2):149-64. doi: 10.2174/1389200013338603. Curr Drug Metab. 2001. PMID: 11469723 Review.
-
The Aryl Hydrocarbon Receptor as an Immune-Modulator of Atmospheric Particulate Matter-Mediated Autoimmunity.Front Immunol. 2018 Dec 6;9:2833. doi: 10.3389/fimmu.2018.02833. eCollection 2018. Front Immunol. 2018. PMID: 30574142 Free PMC article. Review.
Cited by
-
Aryl Hydrocarbon Receptor Role in Co-Ordinating SARS-CoV-2 Entry and Symptomatology: Linking Cytotoxicity Changes in COVID-19 and Cancers; Modulation by Racial Discrimination Stress.Biology (Basel). 2020 Aug 27;9(9):249. doi: 10.3390/biology9090249. Biology (Basel). 2020. PMID: 32867244 Free PMC article. Review.
-
Purpurogallin Protects Keratinocytes from Damage and Apoptosis Induced by Ultraviolet B Radiation and Particulate Matter 2.5.Biomol Ther (Seoul). 2019 Jul 1;27(4):395-403. doi: 10.4062/biomolther.2018.151. Biomol Ther (Seoul). 2019. PMID: 30419635 Free PMC article.
-
Preventive Effect of Pharmaceutical Phytochemicals Targeting the Src Family of Protein Tyrosine Kinases and Aryl Hydrocarbon Receptor on Environmental Stress-Induced Skin Disease.Int J Mol Sci. 2023 Mar 21;24(6):5953. doi: 10.3390/ijms24065953. Int J Mol Sci. 2023. PMID: 36983027 Free PMC article. Review.
-
Glycogen Synthase Kinase 3 Beta Regulates the Human Aryl Hydrocarbon Receptor Cellular Content and Activity.Int J Mol Sci. 2021 Jun 5;22(11):6097. doi: 10.3390/ijms22116097. Int J Mol Sci. 2021. PMID: 34198826 Free PMC article.
-
Stem cell transplantation uncovers TDO-AHR regulation of lung dendritic cells in herpesvirus-induced pathology.JCI Insight. 2021 Jan 25;6(2):e139965. doi: 10.1172/jci.insight.139965. JCI Insight. 2021. PMID: 33491663 Free PMC article.
References
-
- Atkinson RW, Anderson HR, Sunyer J, Ayres J, Baccini M, Vonk JM, Boumghar A, Forastiere F, Forsberg B, Touloumi G, Schwartz J, Katsouyanni K. Acute effects of particulate air pollution on respiratory admissions: results from APHEA 2 project. Air Pollution and Health: a European Approach. Am J Respir Crit Care Med. 2001;164:1860–1866. - PubMed
-
- Ayres JG, Borm P, Cassee FR, Castranova V, Donaldson K, Ghio A, Harrison RM, Hider R, Kelly F, Kooter IM, Marano F, Maynard RL, Mudway I, Nel A, Sioutas C, Smith S, Baeza-Squiban A, Cho A, Duggan S, Froines J. Evaluating the toxicity of airborne particulate matter and nanoparticles by measuring oxidative stress potential--a workshop report and consensus statement. Inhal Toxicol. 2008;20:75–99. - PubMed
-
- Balhara J, Gounni AS. The alveolar macrophages in asthma: a double-edged sword. Mucosal Immunol. 2012;5:605–609. - PubMed
-
- Bein K, Wexler A. A high-efficiency, low-bias method for extracting particulate matter from filter and impactor substrates. Atmos Environ. 2014;90:87–95.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

