Macaque homologs of Kaposi's sarcoma-associated herpesvirus (KSHV) infect germinal center lymphoid cells, epithelial cells in skin and gastrointestinal tract and gonadal germ cells in naturally infected macaques
- PMID: 29689462
- PMCID: PMC5971157
- DOI: 10.1016/j.virol.2018.04.007
Macaque homologs of Kaposi's sarcoma-associated herpesvirus (KSHV) infect germinal center lymphoid cells, epithelial cells in skin and gastrointestinal tract and gonadal germ cells in naturally infected macaques
Abstract
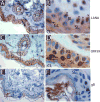
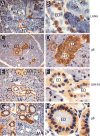
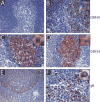
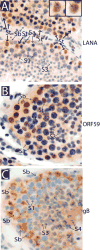
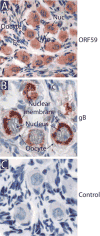
We developed a set of rabbit antisera to characterize infections by the macaque RV2 rhadinovirus homologs of KSHV. We analyzed tissues from rhesus and pig-tailed macaques naturally infected with rhesus rhadinovirus (RRV) or Macaca nemestrina rhadinovirus 2 (MneRV2). Our study demonstrates that RV2 rhadinoviruses have a tropism for epithelial cells, lymphocytes and gonadal germ cells in vivo. We observed latent infections in both undifferentiated and differentiated epithelial cells with expression of the latency marker, LANA. Expression of the early (ORF59) and late (glycoprotein B) lytic markers were detected in highly differentiated cells in epithelial ducts in oral, renal, dermal and gastric mucosal tissue as well as differentiated germ cells in male and female gonads. Our data provides evidence that epithelial and germ cell differentiation in vivo induces rhadinovirus reactivation and suggests that infected epithelial and germ cells play a role in transmission and dissemination of RV2 rhadinovirus infections in vivo.
Keywords: Epithelial; Germ cell; Immunohistochemistry; KSHV; Lymphocyte; Macaque; MneRV2; RRV; Rhadinovirus.
Copyright © 2018 Elsevier Inc. All rights reserved.
Figures


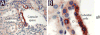
Similar articles
-
The ORF59 DNA polymerase processivity factor homologs of Old World primate RV2 rhadinoviruses are highly conserved nuclear antigens expressed in differentiated epithelium in infected macaques.Virol J. 2009 Nov 18;6:205. doi: 10.1186/1743-422X-6-205. Virol J. 2009. PMID: 19922662 Free PMC article.
-
Complete genome sequence of Pig-tailed macaque rhadinovirus 2 and its evolutionary relationship with rhesus macaque rhadinovirus and human herpesvirus 8/Kaposi's sarcoma-associated herpesvirus.J Virol. 2015 Apr;89(7):3888-909. doi: 10.1128/JVI.03597-14. Epub 2015 Jan 21. J Virol. 2015. PMID: 25609822 Free PMC article.
-
Generation of chimeric forms of rhesus macaque rhadinovirus expressing KSHV envelope glycoproteins gH and gL for utilization in an NHP model of infection.J Virol. 2025 Feb 25;99(2):e0192324. doi: 10.1128/jvi.01923-24. Epub 2025 Jan 21. J Virol. 2025. PMID: 39835812 Free PMC article.
-
Rhesus macaque rhadinovirus-associated disease.Curr Opin Virol. 2013 Jun;3(3):245-50. doi: 10.1016/j.coviro.2013.05.016. Epub 2013 Jun 6. Curr Opin Virol. 2013. PMID: 23747119 Free PMC article. Review.
-
Rhesus monkey rhadinovirus: a model for the study of KSHV.Curr Top Microbiol Immunol. 2007;312:43-69. doi: 10.1007/978-3-540-34344-8_2. Curr Top Microbiol Immunol. 2007. PMID: 17089793 Review.
Cited by
-
Herpesviruses: overview of systematics, genomic complexity and life cycle.Virol J. 2025 May 22;22(1):155. doi: 10.1186/s12985-025-02779-7. Virol J. 2025. PMID: 40399963 Free PMC article. Review.
-
Dangerous Liaisons: Gammaherpesvirus Subversion of the Immunoglobulin Repertoire.Viruses. 2020 Jul 23;12(8):788. doi: 10.3390/v12080788. Viruses. 2020. PMID: 32717815 Free PMC article. Review.
References
-
- Abe Y, Matsubara D, Gatanaga H, Oka S, Kimura S, Sasao Y, Saitoh K, Fujii T, Sato Y, Sata T, Katano H. Distinct expression of Kaposi’s sarcoma-associated herpesvirus-encoded proteins in Kaposi’s sarcoma and multicentric Castleman’s disease. Pathol Int. 2006;56:617–624. - PubMed
-
- Akula SM, Pramod NP, Wang FZ, Chandran B. Integrin alpha3beta1 (CD 49c/29) is a cellular receptor for Kaposi’s sarcoma-associated herpesvirus (KSHV/HHV-8) entry into the target cells. Cell. 2002;108:407–419. - PubMed
-
- Alvisi G, Marin O, Pari G, Mancini M, Avanzi S, Loregian A, Jans DA, Ripalti A. Multiple phosphorylation sites at the C-terminus regulate nuclear import of HCMV DNA polymerase processivity factor ppUL44. Virology. 2011;417:259–267. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

