ZnO Nanostructures for Drug Delivery and Theranostic Applications
- PMID: 29690644
- PMCID: PMC5923598
- DOI: 10.3390/nano8040268
ZnO Nanostructures for Drug Delivery and Theranostic Applications
Abstract
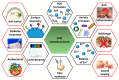
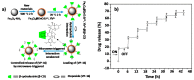
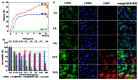
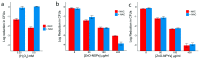
In the last two decades, zinc oxide (ZnO) semiconductor Quantum dots (QDs) have been shown to have fantastic luminescent properties, which together with their low-cost, low-toxicity and biocompatibility have turned these nanomaterials into one of the main candidates for bio-imaging. The discovery of other desirable traits such as their ability to produce destructive reactive oxygen species (ROS), high catalytic efficiency, strong adsorption capability and high isoelectric point, also make them promising nanomaterials for therapeutic and diagnostic functions. Herein, we review the recent progress on the use of ZnO based nanoplatforms in drug delivery and theranostic in several diseases such as bacterial infection and cancer.
Keywords: Quantum dots; ZnO nanoparticles; anti-inflammation; anti-tumour; antibacterial; antifungal; diabetes treatment; drug delivery; theranostic; wound healing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








References
-
- Jia Z., Misra R.D.K. Tunable ZnO quantum dots for bioimaging: synthesis and photoluminescence. Mater. Technol. 2013;28:221–227. doi: 10.1179/1753555713Y.0000000061. - DOI
-
- Volokitin Y., Sinzig J., de Jongh L.J., Schmid G., Vargaftik M.N., Moiseevi I.I. Quantum-size effects in the thermodynamic properties of metallic nanoparticles. Nature. 1996;384:621–623. doi: 10.1038/384621a0. - DOI
-
- Zhang Z.-Y., Xiong H.-M. Photoluminescent ZnO Nanoparticles and Their Biological Applications. Materials. 2015;8:3101–3127. doi: 10.3390/ma8063101. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

