Expression of macrophage migration inhibitory factor in footpad skin lesions with diabetic neuropathy
- PMID: 29690804
- PMCID: PMC5968664
- DOI: 10.1177/1744806918775482
Expression of macrophage migration inhibitory factor in footpad skin lesions with diabetic neuropathy
Abstract
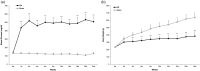
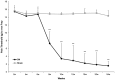
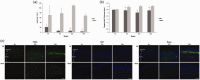
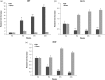
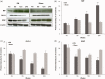
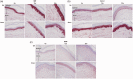
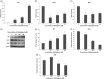
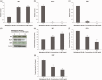
Background Diabetic neuropathy originating in distal lower extremities is associated with pain early in the disease course, overwhelming in the feet. However, the pathogenesis of diabetic neuropathy remains unclear. Macrophage migration inhibitory factor has been implicated in the onset of neuropathic pain and the development of diabetes. Objective of this study was to observe pain syndromes elicited in the footpad of diabetic neuropathy rat model and to assess the contributory role of migration inhibitory factor in the pathogenesis of diabetic neuropathy. Methods Diabetic neuropathy was made in Sprague Dawley rats by streptozotocin. Pain threshold was evaluated using von Frey monofilaments for 24 weeks. On comparable experiment time after streptozotocin injection, all footpads were prepared for following procedures; glutathione assay, terminal deoxynucleotidyl transferase-mediated biotinylated UTP nick end labeling staining, immunohistochemistry staining, real-time reverse transcription polymerase chain reaction, and Western blot. Additionally, human HaCaT skin keratinocytes were treated with methylglyoxal, transfected with migration inhibitory factor/control small interfering RNA, and prepared for real-time reverse transcription polymerase chain reaction and Western blot. Results As compared to sham group, pain threshold was significantly reduced in diabetic neuropathy group, and glutathione was decreased in footpad skin, simultaneously, cell death was increased. Over-expression of migration inhibitory factor, accompanied by low expression of glyoxalase-I and intraepidermal nerve fibers, was shown on the footpad skin lesions of diabetic neuropathy. But, there was no significance in expression of neurotransmitters and inflammatory mediators such as transient receptor potential vanilloid 1, mas-related G protein coupled receptor D, nuclear factor kappa B, tumor necrosis factor-alpha, and interleukin-6 between diabetic neuropathy group and sham group. Intriguingly, small interfering RNA-transfected knockdown of the migration inhibitory factor gene in methylglyoxal-treated skin keratinocytes increased expression of glyoxalase-I and intraepidermal nerve fibers in comparison with control small interfering RNA-transfected cells, which was decreased by induction of methylglyoxal. Conclusions Our findings suggest that migration inhibitory factor can aggravate diabetic neuropathy by suppressing glyoxalase-I and intraepidermal nerve fibers on the footpad skin lesions and provoke pain. Taken together, migration inhibitory factor might offer a pharmacological approach to alleviate pain syndromes in diabetic neuropathy.
Keywords: Diabetic neuropathy; footpad skin; glyoxalase-I; intraepidermal nerve fibers; macrophage migration inhibitory factor; pain.
Figures

References
-
- Boulton AJM Vinik AI Arezzo JC, Bril V, Feldman EL, Freeman R, Malik RA, Maser RE, Sosenko JM and Ziegler D. Diabetic neuropathies: a statement by the American Diabetes Association. Diabetes Care 2005; 28: 956–962. - PubMed
-
- Britland ST Young RJ Sharma AK and Clarke, BF. Association of painful and painless diabetic polyneuropathy with different patterns of nerve fiber degeneration and regeneration. Diabetes 1990; 39: 898–908. - PubMed
-
- Boric M, Skopljanac I, Ferhatovic L, Jelicic Kadic A, Banozic A and Puljak L. Reduced epidermal thickness, nerve degeneration and increased pain-related behavior in rats with diabetes type 1 and 2. J Chem Neuroanat 2013; 53: 33–40. - PubMed
-
- Lauria G, Lombardi R, Borgna M, Penza P, Bianchi R, Savino C, Canta A, Nicolini G, Marmiroli P and Cavaletti G. Intraepidermal nerve fiber density in rat foot pad: neuropathologic-neurophysiologic correlation. J Peripher Nerv Syst 2005; 10: 202–208. - PubMed
-
- Chu CC, Wang SD, Chiang JS and Ho ST. Generalized depletion of free nerve endings and decrease of cutaneous nervous innervation in streptozotocin-induced painful and painless diabetic rats. J Chin Med Assoc 2012; 75: 314–321. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

