The "ComPAS Trial" combined treatment model for acute malnutrition: study protocol for the economic evaluation
- PMID: 29690899
- PMCID: PMC5916722
- DOI: 10.1186/s13063-018-2594-7
The "ComPAS Trial" combined treatment model for acute malnutrition: study protocol for the economic evaluation
Abstract
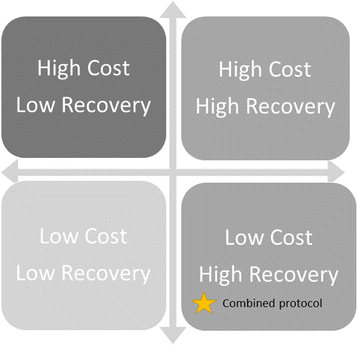
Background: Acute malnutrition is currently divided into severe (SAM) and moderate (MAM) based on level of wasting. SAM and MAM currently have separate treatment protocols and products, managed by separate international agencies. For SAM, the dose of treatment is allocated by the child's weight. A combined and simplified protocol for SAM and MAM, with a standardised dose of ready-to-use therapeutic food (RUTF), is being trialled for non-inferior recovery rates and may be more cost-effective than the current standard protocols for treating SAM and MAM.
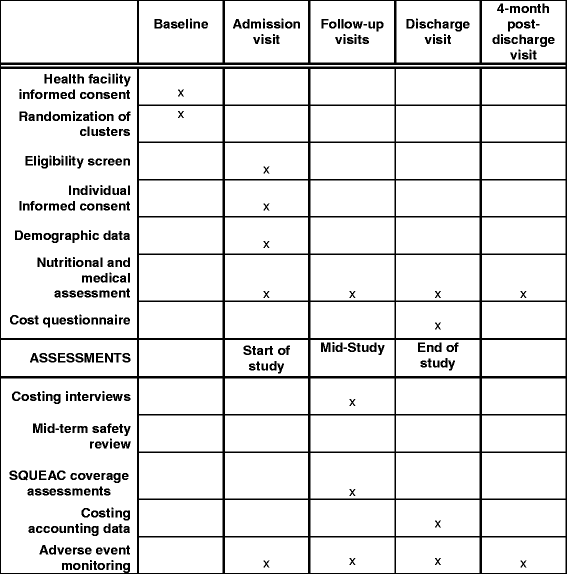
Method: This is the protocol for the economic evaluation of the ComPAS trial, a cluster-randomised controlled, non-inferiority trial that compares a novel combined protocol for treating uncomplicated acute malnutrition compared to the current standard protocol in South Sudan and Kenya. We will calculate the total economic costs of both protocols from a societal perspective, using accounting data, interviews and survey questionnaires. The incremental cost of implementing the combined protocol will be estimated, and all costs and outcomes will be presented as a cost-consequence analysis. Incremental cost-effectiveness ratio will be calculated for primary and secondary outcome, if statistically significant.
Discussion: We hypothesise that implementing the combined protocol will be cost-effective due to streamlined logistics at clinic level, reduced length of treatment, especially for MAM, and reduced dosages of RUTF. The findings of this economic evaluation will be important for policymakers, especially given the hypothesised non-inferiority of the main health outcomes. The publication of this protocol aims to improve rigour of conduct and transparency of data collection and analysis. It is also intended to promote inclusion of economic evaluation in other nutrition intervention studies, especially for MAM, and improve comparability with other studies.
Trial registration: ISRCTN 30393230 , date: 16/03/2017.
Keywords: Community management of acute malnutrition; Cost-consequence analysis; Cost-effectiveness; Moderate acute malnutrition; Severe acute malnutrition.
Conflict of interest statement
Ethics approval and consent to participate
The ComPAS study, including the economic evaluation, was approved by the Kenya Medical Research Institute (KEMRI) (5/1/2017, ref: 551), the Ministry of Health Internal Review Board, South Sudan (21/11/2016), and the London School of Hygiene and Tropical Medicine (28/11/2016, ref: 11826). This protocol, specific to the economic analysis, was reviewed by the International Rescue Committee Institutional Review Board (Ref H1.00.017). Informed written consent is obtained prior to all surveys and interviews (see model consent form in Additional file 2).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- UN General Assembly. Sustainable Development Goals. 2015. http://www.un.org/sustainabledevelopment/sustainable-development-goals/. Accessed 24 Jul 2017.
-
- UNICEF, WHO, The World Bank Group. Joint Child Malnutrition Estimates - Levels and Trends. 2016. http://www.who.int/nutgrowthdb/estimates2016/en/. Accessed 6 Jan 2018.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

