Combined Protocol for Acute Malnutrition Study (ComPAS) in rural South Sudan and urban Kenya: study protocol for a randomized controlled trial
- PMID: 29690916
- PMCID: PMC5978994
- DOI: 10.1186/s13063-018-2643-2
Combined Protocol for Acute Malnutrition Study (ComPAS) in rural South Sudan and urban Kenya: study protocol for a randomized controlled trial
Abstract
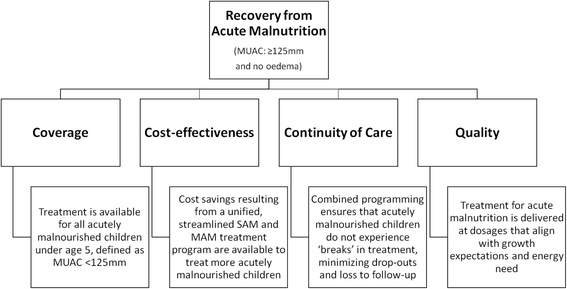
Background: Acute malnutrition is a continuum condition, but severe and moderate forms are treated separately, with different protocols and therapeutic products, managed by separate United Nations agencies. The Combined Protocol for Acute Malnutrition Study (ComPAS) aims to simplify and unify the treatment of uncomplicated severe and moderate acute malnutrition (SAM and MAM) for children 6-59 months into one protocol in order to improve the global coverage, quality, continuity of care and cost-effectiveness of acute malnutrition treatment in resource-constrained settings.
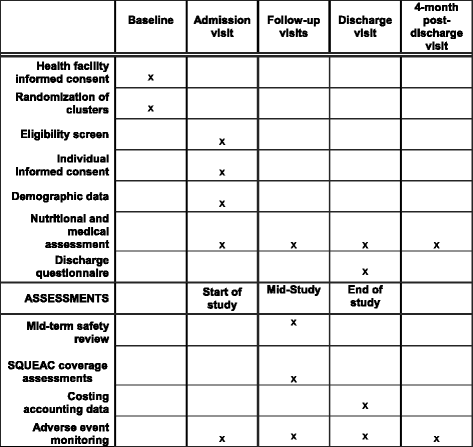
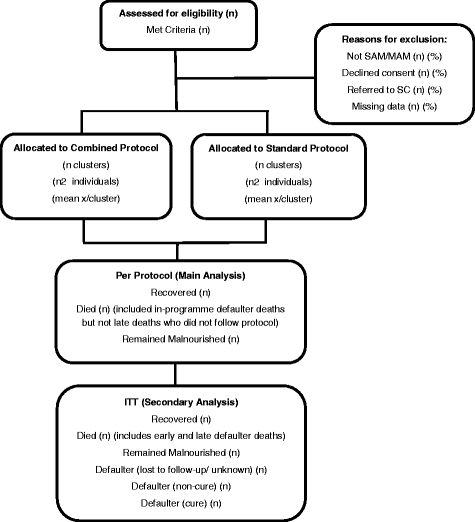
Methods/design: This study is a multi-site, cluster randomized non-inferiority trial with 12 clusters in Kenya and 12 clusters in South Sudan. Participants are 3600 children aged 6-59 months with uncomplicated acute malnutrition. This study will evaluate the impact of a simplified and combined protocol for the treatment of SAM and MAM compared to the standard protocol, which is the national treatment protocol in each country. We will assess recovery rate as a primary outcome and coverage, defaulting, death, length of stay, average weekly weight gain and average weekly mid-upper arm circumference (MUAC) gain as secondary outcomes. Recovery rate is defined across both treatment arms as MUAC ≥125 mm and no oedema for two consecutive visits. Per-protocol and intention-to-treat analyses will be conducted.
Discussion: If the combined protocol is shown to be non-inferior to the standard protocol, updating guidelines to use the combined protocol would eliminate the need for separate products, resources and procedures for MAM treatment. This would likely be more cost-effective, increase availability of services, enable earlier case finding and treatment before deterioration of MAM into SAM, promote better continuity of care and improve community perceptions of the programme.
Trial registration: ISRCTN, ISRCTN30393230 . Registered on 16 March 2017.
Keywords: Acute malnutrition; Cluster randomized trial; Community-based management of acute malnutrition; Kenya; Mid-upper arm circumference; Non-inferiority; Ready-to-use therapeutic food; South Sudan.
Conflict of interest statement
Ethics approval and consent to participate
The ComPAS trial was approved by the Kenya Medical Research Institute (KEMRI) (5 January 2017, ref: 551), the Ministry of Health Internal Review Board, South Sudan (21 November 2016) and the London School of Hygiene and Tropical Medicine, London, UK (28 November 2016, ref: 11826). Informed written consent is obtained prior to all surveys and interviews.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Data and Analytics Section of the Division of Data, Research and Policy, UNICEF; Department of Nutrition for Health and Development, WHO; Development Data Group, World Bank . Levels and trends in child malnutrition: UNICEF-WHO-World Bank Group joint child malnutrition estimates. Washington, D.C.: UNICEF, WHO, World Bank; 2016.
-
- Frison S, et al. Anthropometric indices and measures to assess change in the nutritional status of a population: a systematic literature review. BMC Nutr. 2016;2:76. doi: 10.1186/s40795-016-0104-4. - DOI
-
- The State of Acute Malnutrition. Where are we in treating children with SAM? 2018. http://www.severemalnutrition.org/en/home/Ge. Accessed 23 Feb 2018.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical