Utilization of a Clinical Trial Management System for the Whole Clinical Trial Process as an Integrated Database: System Development
- PMID: 29691212
- PMCID: PMC5941091
- DOI: 10.2196/jmir.9312
Utilization of a Clinical Trial Management System for the Whole Clinical Trial Process as an Integrated Database: System Development
Abstract
Background: Clinical trials pose potential risks in both communications and management due to the various stakeholders involved when performing clinical trials. The academic medical center has a responsibility and obligation to conduct and manage clinical trials while maintaining a sufficiently high level of quality, therefore it is necessary to build an information technology system to support standardized clinical trial processes and comply with relevant regulations.
Objective: The objective of the study was to address the challenges identified while performing clinical trials at an academic medical center, Asan Medical Center (AMC) in Korea, by developing and utilizing a clinical trial management system (CTMS) that complies with standardized processes from multiple departments or units, controlled vocabularies, security, and privacy regulations.
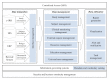
Methods: This study describes the methods, considerations, and recommendations for the development and utilization of the CTMS as a consolidated research database in an academic medical center. A task force was formed to define and standardize the clinical trial performance process at the site level. On the basis of the agreed standardized process, the CTMS was designed and developed as an all-in-one system complying with privacy and security regulations.
Results: In this study, the processes and standard mapped vocabularies of a clinical trial were established at the academic medical center. On the basis of these processes and vocabularies, a CTMS was built which interfaces with the existing trial systems such as the electronic institutional review board health information system, enterprise resource planning, and the barcode system. To protect patient data, the CTMS implements data governance and access rules, and excludes 21 personal health identifiers according to the Health Insurance Portability and Accountability Act (HIPAA) privacy rule and Korean privacy laws. Since December 2014, the CTMS has been successfully implemented and used by 881 internal and external users for managing 11,645 studies and 146,943 subjects.
Conclusions: The CTMS was introduced in the Asan Medical Center to manage the large amounts of data involved with clinical trial operations. Inter- and intraunit control of data and resources can be easily conducted through the CTMS system. To our knowledge, this is the first CTMS developed in-house at an academic medical center side which can enhance the efficiency of clinical trial management in compliance with privacy and security laws.
Keywords: academic medical center; clinical trial; information systems; information technology; privacy.
©Yu Rang Park, Young Jo Yoon, HaYeong Koo, Soyoung Yoo, Chang-Min Choi, Sung-Ho Beck, Tae Won Kim. Originally published in the Journal of Medical Internet Research (http://www.jmir.org), 24.04.2018.
Conflict of interest statement
Conflicts of Interest: None declared
Figures

References
-
- Lu Z, Su J. Clinical data management: Current status, challenges, and future directions from industry perspectives. Open Access J Clin Trials. 2010;2:93–105.
-
- Lu Z. Information technology in pharmacovigilance: Benefits, challenges, and future directions from industry perspectives. Drug Healthc Patient Saf. 2009;1:35–45. http://europepmc.org/abstract/MED/21701609 - PMC - PubMed
-
- Rubin E, Lazar D. AAHCDC. 2009. [2018-02-22]. Clinical Trials Offices: What's New in Research Administration http://www.aahcdc.org/Publications-Resources/Series/Issue-Briefs/View/Ar... .
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

