Suppression of epithelial folding at actomyosin-enriched compartment boundaries downstream of Wingless signalling in Drosophila
- PMID: 29691225
- PMCID: PMC5964650
- DOI: 10.1242/dev.155325
Suppression of epithelial folding at actomyosin-enriched compartment boundaries downstream of Wingless signalling in Drosophila
Abstract
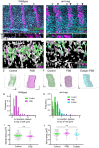
Epithelial folding shapes embryos and tissues during development. Here, we investigate the coupling between epithelial folding and actomyosin-enriched compartmental boundaries. The mechanistic relationship between the two is unclear, because actomyosin-enriched boundaries are not necessarily associated with folds. Also, some cases of epithelial folding occur independently of actomyosin contractility. We investigated the shallow folds called parasegment grooves that form at boundaries between anterior and posterior compartments in the early Drosophila embryo. We demonstrate that formation of these folds requires the presence of an actomyosin enrichment along the boundary cell-cell contacts. These enrichments, which require Wingless signalling, increase interfacial tension not only at the level of the adherens junctions but also along the lateral surfaces. We find that epithelial folding is normally under inhibitory control because different genetic manipulations, including depletion of the Myosin II phosphatase Flapwing, increase the depth of folds at boundaries. Fold depth correlates with the levels of Bazooka (Baz), the Par-3 homologue, along the boundary cell-cell contacts. Moreover, Wingless and Hedgehog signalling have opposite effects on fold depth at the boundary that correlate with changes in Baz planar polarity.
Keywords: Apico-basal polarity; Contractility; Embryo; Epithelium; Morphogenesis; Planar polarity.
© 2018. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

