Undifferentiated Sarcomas in Children Harbor Clinically Relevant Oncogenic Fusions and Gene Copy-Number Alterations: A Report from the Children's Oncology Group
- PMID: 29691299
- PMCID: PMC6335968
- DOI: 10.1158/1078-0432.CCR-18-0672
Undifferentiated Sarcomas in Children Harbor Clinically Relevant Oncogenic Fusions and Gene Copy-Number Alterations: A Report from the Children's Oncology Group
Abstract
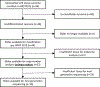
Purpose: A comprehensive analysis of the genomics of undifferentiated sarcomas (UDS) is lacking. We analyzed copy-number alterations and fusion status in patients with UDS prospectively treated on Children's Oncology Group protocol ARST0332.Experimental Design: Copy-number alterations were assessed by OncoScan FFPE Express on 32 UDS. Whole-exome and transcriptome libraries from eight tumors with sufficient archived material were sequenced on HiSeq (2 × 100 bp). Targeted RNA-sequencing using Archer chemistry was performed on two additional cases.Results: Five-year overall survival for patients with UDS was 83% (95% CI, 69%-97%) with risk-adapted therapy (surgery, chemotherapy, and radiotherapy). Both focal and arm-level copy-number alterations were common including gain of 1q (8/32, 25%) and loss of 1p (7/32, 22%), both of which occurred more often in clinically defined high-risk tumors. Tumors with both loss of 1p and gain of 1q carried an especially poor prognosis with a 5-year event-free survival of 20%. GISTIC analysis identified recurrent amplification of FGF1 on 5q31.3 (q = 0.03) and loss of CDKN2A and CDKN2B on 9p21.3 (q = 0.07). Known oncogenic fusions were identified in eight of 10 cases analyzed by next-generation sequencing.Conclusions: Pediatric UDS generally has a good outcome with risk-adapted therapy. A high-risk subset of patients whose tumors have copy-number loss of 1p and gain of 1q was identified with only 20% survival. Oncogenic fusions are common in UDS, and next-generation sequencing should be considered for children with UDS to refine the diagnosis and identify potentially targetable drivers. Clin Cancer Res; 24(16); 3888-97. ©2018 AACR.
©2018 American Association for Cancer Research.
Conflict of interest statement
Conflict of Interest:
TWL has received fees for consulting and advisory board roles from Loxo Oncology, Eli Lilly, and Novartis and TWL’s institution has received research funding from Pfizer. DSH has participated in unpaid medical advisory board (travel expenses reimbursed) for Loxo Oncology, BMS, and Bayer.
All other authors report no conflict of interest.
Figures


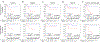
References
-
- Fletcher CDM, Chibon F, Mertens F. Undifferentiated/unclassified sarcomas. In: Fletcher CDM, Bridge JA, Hogendoorn P, Mertens F, eds. WHO Classification of Tumours of Soft Tissue and Bone 4th edition: IARC: Lyon; 2013:236–238.
-
- Sugita S, Arai Y, Tonooka A, et al. A novel CIC-FOXO4 gene fusion in undifferentiated small round cell sarcoma: a genetically distinct variant of Ewing-like sarcoma. Am J Surg Pathol 2014;38(11):1571–1576. - PubMed
-
- Crist WM, Anderson JR, Meza JL, et al. Intergroup rhabdomyosarcoma study-IV: results for patients with nonmetastatic disease. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2001;19(12):3091–3102. - PubMed
-
- Pawel BR, Hamoudi AB, Asmar L, et al. Undifferentiated sarcomas of children: pathology and clinical behavior--an Intergroup Rhabdomyosarcoma study. Med Pediatr Oncol 1997;29(3):170–180. - PubMed
-
- Somers GR, Gupta AA, Doria AS, et al. Pediatric undifferentiated sarcoma of the soft tissues: a clinicopathologic study. Pediatr Dev Pathol 2006;9(2):132–142. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

