Layer-specific morphological and molecular differences in neocortical astrocytes and their dependence on neuronal layers
- PMID: 29691400
- PMCID: PMC5915416
- DOI: 10.1038/s41467-018-03940-3
Layer-specific morphological and molecular differences in neocortical astrocytes and their dependence on neuronal layers
Abstract
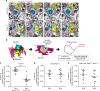
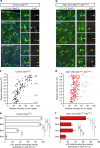
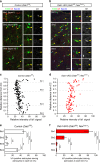
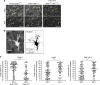
Non-pial neocortical astrocytes have historically been thought to comprise largely a nondiverse population of protoplasmic astrocytes. Here we show that astrocytes of the mouse somatosensory cortex manifest layer-specific morphological and molecular differences. Two- and three-dimensional observations revealed that astrocytes in the different layers possess distinct morphologies as reflected by differences in cell orientation, territorial volume, and arborization. The extent of ensheathment of synaptic clefts by astrocytes in layer II/III was greater than that by those in layer VI. Moreover, differences in gene expression were observed between upper-layer and deep-layer astrocytes. Importantly, layer-specific differences in astrocyte properties were abrogated in reeler and Dab1 conditional knockout mice, in which neuronal layers are disturbed, suggesting that neuronal layers are a prerequisite for the observed morphological and molecular differences of neocortical astrocytes. This study thus demonstrates the existence of layer-specific interactions between neurons and astrocytes, which may underlie their layer-specific functions.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Ramon y Cajal, S. Histology of the Nervous System of Man and Vertebrates (Oxford University Press, New York, 1897).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

