Biomechanics and mechanical signaling in the ovary: a systematic review
- PMID: 29691711
- PMCID: PMC6063820
- DOI: 10.1007/s10815-018-1180-y
Biomechanics and mechanical signaling in the ovary: a systematic review
Abstract
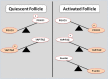
Purpose: Mammalian oogenesis and folliculogenesis share a dynamic connection that is critical for gamete development. For maintenance of quiescence or follicular activation, follicles must respond to soluble signals (growth factors and hormones) and physical stresses, including mechanical forces and osmotic shifts. Likewise, mechanical processes are involved in cortical tension and cell polarity in oocytes. Our objective was to examine the contribution and influence of biomechanical signaling in female mammalian gametogenesis.
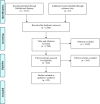
Methods: We performed a systematic review to assess and summarize the effects of mechanical signaling and mechanotransduction in oocyte maturation and folliculogenesis and to explore possible clinical applications. The review identified 2568 publications of which 122 met the inclusion criteria.
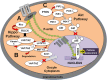
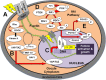
Results: The integration of mechanical and cell signaling pathways in gametogenesis is complex. Follicular activation or quiescence are influenced by mechanical signaling through the Hippo and Akt pathways involving the yes-associated protein (YAP), transcriptional coactivator with PDZ-binding motif (TAZ), phosphatase and tensin homolog deleted from chromosome 10 (PTEN) gene, the mammalian target of rapamycin (mTOR), and forkhead box O3 (FOXO3) gene.
Conclusions: There is overwhelming evidence that mechanical signaling plays a crucial role in development of the ovary, follicle, and oocyte throughout gametogenesis. Emerging data suggest the complexities of mechanotransduction and the biomechanics of oocytes and follicles are integral to understanding of primary ovarian insufficiency, ovarian aging, polycystic ovary syndrome, and applications of fertility preservation.
Keywords: Folliculogenesis; Mechanical signaling; Mechanotransduction; Oocyte maturation; Ovarian biomechanics.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Beyond apoptosis: evidence of other regulated cell death pathways in the ovary throughout development and life.Hum Reprod Update. 2023 Jul 5;29(4):434-456. doi: 10.1093/humupd/dmad005. Hum Reprod Update. 2023. PMID: 36857094 Free PMC article.
-
N-cadherin mechanosensing in ovarian follicles controls oocyte maturation and ovulation.Elife. 2025 Jul 29;13:RP92068. doi: 10.7554/eLife.92068. Elife. 2025. PMID: 40728874 Free PMC article.
-
Early signaling pathways during in vitro culture of isolated primordial follicles.Mol Hum Reprod. 2025 Jul 3;31(3):gaaf026. doi: 10.1093/molehr/gaaf026. Mol Hum Reprod. 2025. PMID: 40489658
-
Aromatase inhibitors (letrozole) for subfertile women with polycystic ovary syndrome.Cochrane Database Syst Rev. 2018 May 24;5(5):CD010287. doi: 10.1002/14651858.CD010287.pub3. Cochrane Database Syst Rev. 2018. Update in: Cochrane Database Syst Rev. 2022 Sep 27;9:CD010287. doi: 10.1002/14651858.CD010287.pub4. PMID: 29797697 Free PMC article. Updated.
-
Individualised gonadotropin dose selection using markers of ovarian reserve for women undergoing in vitro fertilisation plus intracytoplasmic sperm injection (IVF/ICSI).Cochrane Database Syst Rev. 2018 Feb 1;2(2):CD012693. doi: 10.1002/14651858.CD012693.pub2. Cochrane Database Syst Rev. 2018. Update in: Cochrane Database Syst Rev. 2024 Jan 4;1:CD012693. doi: 10.1002/14651858.CD012693.pub3. PMID: 29388198 Free PMC article. Updated.
Cited by
-
Longevity pathways are associated with human ovarian ageing.Hum Reprod Open. 2021 May 16;2021(2):hoab020. doi: 10.1093/hropen/hoab020. eCollection 2021. Hum Reprod Open. 2021. PMID: 34027130 Free PMC article.
-
Ovarian stiffness increases with age in the mammalian ovary and depends on collagen and hyaluronan matrices.Aging Cell. 2020 Nov;19(11):e13259. doi: 10.1111/acel.13259. Epub 2020 Oct 20. Aging Cell. 2020. PMID: 33079460 Free PMC article.
-
Ultrasound Shear Wave Velocity Varies Across Anatomical Region in Ex Vivo Bovine Ovaries.Tissue Eng Part A. 2020 Jul;26(13-14):720-732. doi: 10.1089/ten.tea.2020.0037. Epub 2020 Jun 30. Tissue Eng Part A. 2020. PMID: 32609070 Free PMC article.
-
Maternal effect factors that contribute to oocytes developmental competence: an update.J Assist Reprod Genet. 2022 Apr;39(4):861-871. doi: 10.1007/s10815-022-02434-y. Epub 2022 Feb 15. J Assist Reprod Genet. 2022. PMID: 35165782 Free PMC article. Review.
-
Hippo signaling in the ovary and polycystic ovarian syndrome.J Assist Reprod Genet. 2018 Oct;35(10):1763-1771. doi: 10.1007/s10815-018-1235-0. Epub 2018 Aug 17. J Assist Reprod Genet. 2018. PMID: 30120633 Free PMC article. Review.
References
-
- Ingber DE. Cellular mechanotransduction: putting all the pieces together again. FASEB J. 2006;20:1230–1232. - PubMed
-
- Heisenberg CP, Bellaïche Y. Forces in tissue morphogenesis and patterning. Cell. 2013;153:948–962. - PubMed
-
- Albertini DF, Barrett SL. The developmental origins of mammalian oocyte polarity. Semin Cell Dev Biol. 2004;15:599–606. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

