Rufinamide add-on therapy for refractory epilepsy
- PMID: 29691835
- PMCID: PMC6494418
- DOI: 10.1002/14651858.CD011772.pub2
Rufinamide add-on therapy for refractory epilepsy
Update in
-
Rufinamide add-on therapy for drug-resistant epilepsy.Cochrane Database Syst Rev. 2020 Nov 8;11(11):CD011772. doi: 10.1002/14651858.CD011772.pub3. Cochrane Database Syst Rev. 2020. PMID: 33179247 Free PMC article.
Abstract
Background: Epilepsy is a central nervous system disorder (neurological disorder). Epileptic seizures are the result of excessive and abnormal cortical nerve cell electrical activity in the brain. Despite the development of more than 10 new antiepileptic drugs (AEDs) since the early 2000s, approximately a third of people with epilepsy remain resistant to pharmacotherapy, often requiring treatment with a combination of AEDs. In this review, we summarised the current evidence regarding rufinamide, a novel anticonvulsant medication, which, as a triazole derivative, is structurally unrelated to any other currently used anticonvulsant medication, when used as an add-on treatment for refractory epilepsy. In January 2009, rufinamide was approved by the US Food and Drug Administration for treatment of children four years of age and older with Lennox-Gastaut syndrome. It is also approved as an add-on treatment for adults and adolescents with focal seizures.
Objectives: To evaluate the efficacy and tolerability of rufinamide when used as an add-on treatment in people with refractory epilepsy.
Search methods: On 2 October 2017, we searched the Cochrane Epilepsy Group Specialized Register, the Cochrane Central Register of Controlled Trials (CENTRAL) via the Cochrane Register of Studies Online (CRSO), MEDLINE (Ovid, 1946), ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP). We imposed no language restrictions. We also contacted the manufacturers of rufinamide and authors in the field to identify any relevant unpublished studies.
Selection criteria: Randomised, double-blind, placebo-controlled, add-on trials of rufinamide, recruiting people (of any age or gender) with refractory epilepsy.
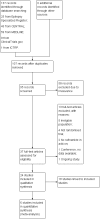
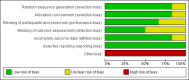
Data collection and analysis: Two review authors independently selected trials for inclusion and extracted the relevant data. We assessed the following outcomes: 50% or greater reduction in seizure frequency (primary outcomes); seizure freedom; treatment withdrawal; and adverse effects (secondary outcomes). Primary analyses were intention-to-treat (ITT) and we presented summary risk ratios (RR) with 95% confidence intervals (CI). We evaluated dose response in regression models. We carried out a risk of bias assessment for each included study using the Cochrane 'Risk of bias' tool and assessed the overall quality of evidence using the GRADE approach, which we presented in a 'Summary of findings' table.
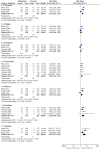
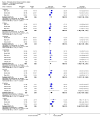
Main results: The review included six trials, representing 1759 participants. Four trials (1563 participants) included people with uncontrolled focal seizures. Two trials (196 participants) included established Lennox-Gastaut syndrome. Overall, the age of the adults ranged from 18 to 80 years and the age of the infants ranged from four to 16 years. Baseline phase ranged from 28 to 56 days and double-blind phases from 84 to 96 days. Five of the six included trials described adequate methods of concealment of randomisation and only three described adequate blinding. All analyses were by ITT. Overall, five studies were at low risk of bias, and one had unclear risk of bias due to lack of reported information around study design. All trials were sponsored by the manufacturer of rufinamide, and therefore, were at high risk of funding bias.The overall RR for 50% or greater reduction in seizure frequency was 1.79 (95% CI 1.44 to 2.22; 6 RCTs; moderate-quality evidence) indicating that rufinamide (plus conventional AED) was significantly more effective than placebo (plus conventional AED) in reducing seizure frequency by at least 50%, when added to conventionally used AEDs in people with refractory focal epilepsy. The overall RR for treatment withdrawal (for any reason and due to AED) was 1.83 (95% CI 1.45 to 2.31; 6 RCTs; moderate-quality evidence) showing that rufinamide was significantly more likely to be withdrawn than placebo. In respect of adverse effects, most were significantly more likely to occur in the rufinamide-treated group. The adverse events significantly associated with rufinamide were: headache, dizziness, somnolence, vomiting, nausea, fatigue and diplopia. The RRs of these adverse effects were: headache 1.36 (95% Cl 1.08 to 1.69; 3 RCTs; high-quality evidence); dizziness 2.52 (95% Cl 1.90 to 3.34; 3 RCTs; moderate-quality evidence); somnolence 1.94 (95% Cl 1.44 to 2.61; 6 RCTs; moderate-quality evidence); vomiting 2.95 (95% Cl 1.80 to 4.82; 4 RCTs; low-quality evidence); nausea 1.87 (95% Cl 1.33 to 2.64; 3 RCTs; moderate-quality evidence); fatigue 1.46 (95% Cl 1.08 to 1.97; 3 RCTs; moderate-quality evidence); and diplopia 4.60 (95% Cl 2.53 to 8.38; 3 RCTs; low-quality evidence). There was no important heterogeneity between studies for any of the outcomes. Overall, we assessed the evidence as moderate to low quality, due to potential risk of bias from some studies contributing to the analysis and wide CIs.
Authors' conclusions: In people with drug-resistant focal epilepsy, rufinamide when used as an add-on treatment was effective in reducing seizure frequency. However, the trials reviewed were of relatively short duration and provided no evidence for the long-term use of rufinamide. In the short term, rufinamide as an add-on was associated with several adverse events. This review focused on the use of rufinamide in drug-resistant focal epilepsy and the results cannot be generalised to add-on treatment for generalised epilepsies. Likewise, no inference can be made about the effects of rufinamide when used as monotherapy.
Conflict of interest statement
MP: none known.
AGM: a consortium of pharmaceutical companies (GSK, Eisai, UCB Pharma) funded the National Audit of Seizure Management in Hospitals (NASH) through grants paid to the University of Liverpool. Professor Tony Marson is part funded by National Institute for Health Research Collaboration for Leadership in Applied Health Research and Care North West Coast (NIHR CLAHRC NWC).
HP: none known.
Figures






Similar articles
-
Rufinamide add-on therapy for drug-resistant epilepsy.Cochrane Database Syst Rev. 2020 Nov 8;11(11):CD011772. doi: 10.1002/14651858.CD011772.pub3. Cochrane Database Syst Rev. 2020. PMID: 33179247 Free PMC article.
-
Pregabalin add-on for drug-resistant focal epilepsy.Cochrane Database Syst Rev. 2022 Mar 29;3(3):CD005612. doi: 10.1002/14651858.CD005612.pub5. Cochrane Database Syst Rev. 2022. PMID: 35349176 Free PMC article.
-
Lamotrigine add-on for drug-resistant partial epilepsy.Cochrane Database Syst Rev. 2016 Jun 22;2016(6):CD001909. doi: 10.1002/14651858.CD001909.pub2. Cochrane Database Syst Rev. 2016. Update in: Cochrane Database Syst Rev. 2020 Mar 20;3:CD001909. doi: 10.1002/14651858.CD001909.pub3. PMID: 27329345 Free PMC article. Updated.
-
Levetiracetam add-on for drug-resistant focal epilepsy: an updated Cochrane Review.Cochrane Database Syst Rev. 2012 Sep 12;2012(9):CD001901. doi: 10.1002/14651858.CD001901.pub2. Cochrane Database Syst Rev. 2012. PMID: 22972056 Free PMC article.
-
Immunomodulatory interventions for focal epilepsy.Cochrane Database Syst Rev. 2023 Oct 16;10(10):CD009945. doi: 10.1002/14651858.CD009945.pub3. Cochrane Database Syst Rev. 2023. PMID: 37842826 Free PMC article.
Cited by
-
Adjunctive Rufinamide in Children with Lennox-Gastaut Syndrome: A Literature Review.Neuropsychiatr Dis Treat. 2020 Feb 5;16:369-379. doi: 10.2147/NDT.S185774. eCollection 2020. Neuropsychiatr Dis Treat. 2020. PMID: 32103957 Free PMC article. Review.
-
Elucidating the Potential Side Effects of Current Anti-Seizure Drugs for Epilepsy.Curr Neuropharmacol. 2021;19(11):1865-1883. doi: 10.2174/1570159X19666210826125341. Curr Neuropharmacol. 2021. PMID: 34525933 Free PMC article. Review.
-
New Methods Used in Pharmacokinetics and Therapeutic Monitoring of the First and Newer Generations of Antiepileptic Drugs (AEDs).Molecules. 2020 Nov 2;25(21):5083. doi: 10.3390/molecules25215083. Molecules. 2020. PMID: 33147810 Free PMC article. Review.
-
Rufinamide add-on therapy for drug-resistant epilepsy.Cochrane Database Syst Rev. 2020 Nov 8;11(11):CD011772. doi: 10.1002/14651858.CD011772.pub3. Cochrane Database Syst Rev. 2020. PMID: 33179247 Free PMC article.
-
Effect of Ibuprofen on Autophagy of Astrocytes During Pentylenetetrazol-Induced Epilepsy and its Significance: An Experimental Study.Neurochem Res. 2019 Nov;44(11):2566-2576. doi: 10.1007/s11064-019-02875-5. Epub 2019 Sep 18. Neurochem Res. 2019. PMID: 31535354
References
References to studies included in this review
-
- Biton V, Krauss G, Vasquez‐Santana B, Bibbiani F, Mann A, Perdomo C, et al. A randomized, double‐blind, placebo‐controlled, parallel‐group study of rufinamide as adjunctive therapy for refractory partial‐onset seizures. Epilepsia 2011;52(2):234‐42. [DOI: 10.1111/j.1528-1167.2010.02729.x; PUBMED: 20887365] - DOI - PubMed
- NCT00334958. Rufinamide Given as Adjunctive Therapy in Patients With Refractory Partial Seizures. https://ClinicalTrials.gov/show/NCT003349582006.
-
- Aldenkamp AP, Alpherts WCJ. The effect of the new antiepileptic drug rufinamide on cognitive functions. Epilepsia 2006;47(7):1153‐9. [DOI: 10.1111/j.1528-1167.2006.00589.x; ISSN: 0013‐9580; PUBMED: 16886978] - DOI - PubMed
- Brodie MJ, Rosenfeld W, Vasquez B, Sachdeo R, Ko D, Perdomo C, et al. Efficacy and safety of rufinamide as adjunctive therapy in adult patients with inadequately controlled partial seizures. Epilepsia 2005;46 Suppl 8:171, Abstract no: 2.239. - PubMed
- Brodie MJ, Rosenfeld W, Vazquez B, Sachdeo R, Perdomo C, Mann A, et al. Rufinamide for the adjunctive treatment of partial seizures in adults and adolescents: a randomized placebo‐controlled trial. Epilepsia 2009;50(8):1899‐909. [DOI: 10.1111/j.1528-1167.2009.02160.x; PUBMED: 19490053] - DOI - PubMed
- Rosenfeld W, Brodie M, Vazquez B, Sachdeo R, Ko D, Perdomo C, et al. Efficacy and safety of rufinamide as adjunctive therapy in adult patients with inadequately controlled partial seizures. Epilepsia 2006;47 Suppl 3:140, Abstract no: p538.
- Vazquez BR, Sachdeo R, Maxoutova AL, Beydoun A, Karolchyk MA, Mesenbrink PG. Efficacy and safety of rufinamide as adjunctive therapy in adult patients with therapy‐resistant partial‐onset seizures. Epilepsia 2000;41 Suppl 7:255, Abstract no: L.09.
-
- Elger C, Stefan H, Perdomo C, Arroyo S. Dose‐range relationships of rufinamide in patients with inadequately controlled partial seizures. Epilepsia 2005;46 Suppl 8:83‐4, Abstract no: 1.201.
- Elger C, Stefan H, Perdomo C, Arroyo S. Dose‐range relationships of rufinamide in patients with inadequately controlled partial seizures. Epilepsia 2006;47 Suppl 3:140, Abstract no: p539.
- Elger CE, Stefan H, Mann A, Narurkar M, Sun Y, Perdomo C. A 24‐week multicenter, randomized, double‐blind, parallel‐group, dose ranging study of rufinamide in adults and adolescents with inadequately controlled partial seizures. Epilepsy Research 2010;88(2‐3):255‐63. [DOI: 10.1016/j.eplepsyres.2009.12.003; PUBMED: 20061123] - DOI - PubMed
- Stefan H, Remy C, Guberman A, Thomson A, Arbelaiz R, Karolchyk MA. A multicenter double‐blind randomized placebo‐controlled 5‐arm, parallel‐group trial of rufinamide in patients with therapy‐resistant partial seizures. Epilepsia 2000;41 Suppl Florence:39. - PubMed
-
- Glauser T, Arzimanoglou A, Litzinger M, Frank LM, Vigevano F, Perdomo C. Efficacy and safety of rufinamide as adjunctive therapy for inadequately controlled partial seizures in pediatric patients. Epilepsia 2005;46 Suppl 8:194‐5.
-
- Glauser T, Kluger G, Krauss G, Arroyo S. Effects of rufinamide on the frequency of different seizure types in patients with Lennox‐Gastaut syndrome: a long‐term study. Epilepsia 2007;48 Suppl 7:156, Abstract no: p415.
- Glauser T, Kluger G, Sachdeo R, Krauss G, Perdomo C, Arroyo S. Rufinamide for generalized seizures associated with Lennox‐Gastaut Syndrome. Neurology 2008;70(21):1950‐8. [DOI: 10.1212/01.wnl.0000303813.95800.0d; PUBMED: 18401024] - DOI - PubMed
- Glauser T, Kluger G, Sachdeo R, Krauss G, Perdomo C, Arroyo S. Short‐term and long‐term efficacy and safety of rufinamide as adjunctive therapy in patients with inadequately controlled Lennox‐Gastaut syndrome. Annals of neurology 2005;58 Suppl 9:S92, Abstract no: M15.
- Glauser T, Kluger G, Sachedo R, Krauss G, Perdomo C, Arroyo S. Efficacy and safety of rufinamide adjunctive therapy in patients with Lennox‐Gastaut syndrome (LGS): a multicenter, randomized, double‐blind, placebo‐controlled, parallel trial. Neurology 2005;64(10):1826, Abstract no: LBS.001.
- Glauser T, Santana BV, Wang W, Narurkar M. Early and sustained response to rufinamide as adjunctive therapy for seizures associated with Lennox‐Gastaut syndrome. Epilepsia 2009;50 Suppl 11:261, Abstract no: 2.229.
- Kluger G, Glauser T, Sachdeo R, Krauss G, Perdomo C, Arroyo S. Short‐term and long‐term efficacy and safety of rufinamide as adjunctive therapy in patients with inadequately controlled Lennox‐Gastaut syndrome. Epilepsia 2006;47 Suppl 3:139, Abstract no: p537.
- Krauss GL, Glauser T, Kluger G, Arroyo S. Long‐term safety of rufinamide in patients with Lennox‐Gastaut syndrome. Epilepsia 2007;48 Suppl 6:359, Abstract no: 3.303. - PubMed
- McMurray R, Striano P. Treatment of Adults with Lennox‐Gastaut Syndrome: Further Analysis of Efficacy and Safety/Tolerability of Rufinamide. Neurology & Therapy 2016;5(1):35‐43. [DOI: 10.1007/s40120-016-0041-9; ISSN: 2193‐8253 2193‐6536; PUBMED: 26861566] - DOI - PMC - PubMed
References to studies excluded from this review
-
- Benedict A, Verdian L, Maclaine G. The cost effectiveness of rufinamide in the treatment of Lennox‐Gastaut syndrome in the UK. Pharmacoeconomics 2010;28(3):185‐99. - PubMed
-
- Biton V, Sachdeo R, Rosenfeld W, Schachter S, Perdomo C, Arroyo S. Efficacy and safety of adjunctive rufinamide in patients with inadequately controlled primary generalized tonic‐clonic seizures. Epilepsia 2005;46(8):206.
-
- Critchley D, Fuseau E, Perdomo C, Arroyo S. Pharmacokinetic and pharmacodynamic parameters of adjunctive rufinamide in patients with Lennox‐Gastaut syndrome. Epilepsia 2005;46 Suppl 8:209.
-
- Kim SH, Eun SH, Kang HC, Kwon EJ, Byeon JH, Lee YM. Rufinamide as an adjuvant treatment in children with Lennox‐Gastaut syndrome. Seizure 2012;21(4):288‐91. - PubMed
References to ongoing studies
-
- Arzimanoglou A, Ferreira JA, Satlin A, Mendes S, Williams B, Critchley D, et al. Safety and pharmacokinetic profile of rufinamide in pediatric patients aged less than 4 years with Lennox‐Gastaut syndrome: an interim analysis from a multicenter, randomized, active‐controlled, open‐label study. European Journal of Paediatric Neurology: EJPN 2016;20(3):393‐402. - PubMed
Additional references
-
- Aldenkamp AP, Alpherts WCJ. The effect of the new antiepileptic drug rufinamide on cognitive functions. Epilepsia 2006;47(7):1153‐9. - PubMed
-
- Brodie MJ, Rosenfels W, Vasquez B, Sachdeo R, Perdomo C. Efficacy and safety of rufinamide as adjunctive therapy in adult patients with inadequately controlled partial seizures. Epilepsia 2005;46 Suppl 8:171. - PubMed
-
- Elger C, Stefan H, Perdomo C, Arroyo S. Dose‐range relationships of rufinamide in patients with inadequately controlled partial seizures. Epilepsia 2005;46 Suppl 8:83‐4.
-
- Elger C, Stefan H, Perdomo C, Arroyo S. Dose‐range relationships of rufinamide in patients with inadequately controlled partial seizures. Epilepsia 2006;47 Suppl 3:140.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

