Functional Studies of T Regulatory Lymphocytes in Human Schistosomiasis in Western Kenya
- PMID: 29692308
- PMCID: PMC6086154
- DOI: 10.4269/ajtmh.17-0966
Functional Studies of T Regulatory Lymphocytes in Human Schistosomiasis in Western Kenya
Abstract
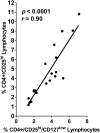
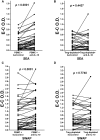
Immunoregulation is considered a common feature of Schistosoma mansoni infections, and elevated levels of T regulatory (Treg) lymphocytes have been reported during chronic human schistosomiasis. We now report that the removal of Treg (CD4+/CD25hi/CD127low lymphocytes) from peripheral blood mononuclear cells (PBMCs) of S. mansoni-infected individuals leads to increased levels of phytohemagglutinin (PHA)-stimulated interferon gamma (IFNγ) production and decreased interleukin-10 (IL-10) responses. Exposure to schistosome antigens did not result in measurable IFNγ by either PBMC or Treg-depleted populations. Interleukin-10 responses to soluble egg antigens (SEA) by PBMC were unchanged by Treg depletion, but the depletion of Treg greatly decreased IL-10 production to soluble worm antigenic preparation (SWAP). Proliferative responses to PHA increased upon Treg removal, but responses to SEA or SWAP did not, unless only initially low responders were evaluated. Addition of anti-IL-10 increased PBMC proliferative responses to either SEA or SWAP, but did not alter responses by Treg-depleted cells. Blockade by anti-transforming growth factor-beta (TGF-β) increased SEA but not SWAP proliferative responses by PBMC, whereas anti-TGF-β increased both SEA- and SWAP-stimulated responses by Treg-depleted cultures. Addition of both anti-IL-10 and anti-TGF-β to PBMC or Treg-depleted populations increased proliferation of both populations to either SEA or SWAP. These studies demonstrate that Treg appear to produce much of the antigen-stimulated IL-10, but other cell types or subsets of Treg may produce much of the TGF-β. The elevated levels of Treg seen in chronic schistosomiasis appear functional and involve IL-10 and TGF-β in antigen-specific immunoregulation perhaps leading to regulation of immunopathology and/or possibly decreased immunoprotective responses.
Figures







Similar articles
-
Cytokine production in acute versus chronic human Schistosomiasis mansoni: the cross-regulatory role of interferon-gamma and interleukin-10 in the responses of peripheral blood mononuclear cells and splenocytes to parasite antigens.J Infect Dis. 1999 Jun;179(6):1502-14. doi: 10.1086/314748. J Infect Dis. 1999. PMID: 10228073
-
[Activities of treg cells stimulated by soluble adult worm antigen and egg antigen of Schistosoma japonicum].Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi. 2013 Apr;25(2):146-50. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi. 2013. PMID: 23894834 Chinese.
-
Cytokine regulation of human immune response to Schistosoma mansoni: analysis of the role of IL-4, IL-5 and IL-10 on peripheral blood mononuclear cell responses.Scand J Immunol. 1997 Oct;46(4):393-8. doi: 10.1046/j.1365-3083.1997.d01-136.x. Scand J Immunol. 1997. PMID: 9350291
-
Cytokines as determinants of resistance and pathology in human Schistosoma mansoni infection.Braz J Med Biol Res. 1998 Jan;31(1):171-7. doi: 10.1590/s0100-879x1998000100024. Braz J Med Biol Res. 1998. PMID: 9686196 Review.
-
Immunological profiles of patients from endemic areas infected with Schistosoma mansoni.Mem Inst Oswaldo Cruz. 1992;87 Suppl 4:139-42. doi: 10.1590/s0074-02761992000800020. Mem Inst Oswaldo Cruz. 1992. PMID: 1343884 Review.
Cited by
-
Adults from Kisumu, Kenya have robust γδ T cell responses to Schistosoma mansoni, which are modulated by tuberculosis.PLoS Negl Trop Dis. 2020 Oct 12;14(10):e0008764. doi: 10.1371/journal.pntd.0008764. eCollection 2020 Oct. PLoS Negl Trop Dis. 2020. PMID: 33044959 Free PMC article.
-
Schistosoma japonicum Infection in Treg-Specific USP21 Knockout Mice.J Immunol Res. 2021 Feb 9;2021:6613162. doi: 10.1155/2021/6613162. eCollection 2021. J Immunol Res. 2021. PMID: 33628844 Free PMC article.
-
Promising Technologies in the Field of Helminth Vaccines.Front Immunol. 2021 Aug 19;12:711650. doi: 10.3389/fimmu.2021.711650. eCollection 2021. Front Immunol. 2021. PMID: 34489961 Free PMC article. Review.
-
Evaluation of IL-35, as a Possible Biomarker for Follow-Up after Therapy, in Chronic Human Schistosoma Infection.Vaccines (Basel). 2023 May 17;11(5):995. doi: 10.3390/vaccines11050995. Vaccines (Basel). 2023. PMID: 37243099 Free PMC article.
-
Schistosome Infection and Schistosome-Derived Products as Modulators for the Prevention and Alleviation of Immunological Disorders.Front Immunol. 2021 Feb 22;12:619776. doi: 10.3389/fimmu.2021.619776. eCollection 2021. Front Immunol. 2021. PMID: 33692793 Free PMC article. Review.
References
-
- Joseph S, et al. 2004. Increases in human T helper 2 cytokine responses to Schistosoma mansoni worm and worm‐tegument antigens are induced by treatment with praziquantel. J Infect Dis 190: 835–842. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

