A defect in KCa3.1 channel activity limits the ability of CD8+ T cells from cancer patients to infiltrate an adenosine-rich microenvironment
- PMID: 29692361
- PMCID: PMC6006512
- DOI: 10.1126/scisignal.aaq1616
A defect in KCa3.1 channel activity limits the ability of CD8+ T cells from cancer patients to infiltrate an adenosine-rich microenvironment
Abstract
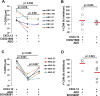
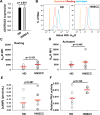
The limited ability of cytotoxic T cells to infiltrate solid tumors hampers immune surveillance and the efficacy of immunotherapies in cancer. Adenosine accumulates in solid tumors and inhibits tumor-specific T cells. Adenosine inhibits T cell motility through the A2A receptor (A2AR) and suppression of KCa3.1 channels. We conducted three-dimensional chemotaxis experiments to elucidate the effect of adenosine on the migration of peripheral blood CD8+ T cells from head and neck squamous cell carcinoma (HNSCC) patients. The chemotaxis of HNSCC CD8+ T cells was reduced in the presence of adenosine, and the effect was greater on HNSCC CD8+ T cells than on healthy donor (HD) CD8+ T cells. This response correlated with the inability of CD8+ T cells to infiltrate tumors. The effect of adenosine was mimicked by an A2AR agonist and prevented by an A2AR antagonist. We found no differences in A2AR expression, 3',5'-cyclic adenosine monophosphate abundance, or protein kinase A type 1 activity between HNSCC and HD CD8+ T cells. We instead detected a decrease in KCa3.1 channel activity, but not expression, in HNSCC CD8+ T cells. Activation of KCa3.1 channels by 1-EBIO restored the ability of HNSCC CD8+ T cells to chemotax in the presence of adenosine. Our data highlight the mechanism underlying the increased sensitivity of HNSCC CD8+ T cells to adenosine and the potential therapeutic benefit of KCa3.1 channel activators, which could increase infiltration of these T cells into tumors.
Copyright © 2018 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Figures

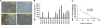

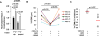
References
-
- De Meulenaere A, Vermassen T, Aspeslagh S, Zwaenepoel K, Deron P, Duprez F, Rottey S, Ferdinande L. Prognostic markers in oropharyngeal squamous cell carcinoma: focus on CD70 and tumour infiltrating lymphocytes. Pathology. 2017;49:397–404. - PubMed
-
- Kershaw MH, Westwood JA, Darcy KP. Gene-engineered T cells for cancer therapy. Nat Rev Cancer. 2013;13:525–541. - PubMed
-
- Beavis PA, Milenkovski N, Henderson MA, John LB, Allard B, Loi S, Kershaw MH, Stagg J, Darcy PK. Adenosine Receptor 2A Blockade Increases the Efficacy of Anti-PD-1 through Enhanced Antitumor T-cell Responses. Cancer Immunol Res. 2015;3:506–517. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

