Clostridium difficile - From Colonization to Infection
- PMID: 29692762
- PMCID: PMC5902504
- DOI: 10.3389/fmicb.2018.00646
Clostridium difficile - From Colonization to Infection
Abstract
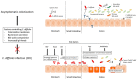
Clostridium difficile is the most frequent cause of nosocomial antibiotic-associated diarrhea. The incidence of C. difficile infection (CDI) has been rising worldwide with subsequent increases in morbidity, mortality, and health care costs. Asymptomatic colonization with C. difficile is common and a high prevalence has been found in specific cohorts, e.g., hospitalized patients, adults in nursing homes and in infants. However, the risk of infection with C. difficile differs significantly between these cohorts. While CDI is a clear indication for therapy, colonization with C. difficile is not believed to be a direct precursor for CDI and therefore does not require treatment. Antibiotic therapy causes alterations of the intestinal microbial composition, enabling C. difficile colonization and consecutive toxin production leading to disruption of the colonic epithelial cells. Clinical symptoms of CDI range from mild diarrhea to potentially life-threatening conditions like pseudomembranous colitis or toxic megacolon. While antibiotics are still the treatment of choice for CDI, new therapies have emerged in recent years such as antibodies against C. difficile toxin B and fecal microbial transfer (FMT). This specific therapy for CDI underscores the role of the indigenous bacterial composition in the prevention of the disease in healthy individuals and its role in the pathogenesis after alteration by antibiotic treatment. In addition to the pathogenesis of CDI, this review focuses on the colonization of C. difficile in the human gut and factors promoting CDI.
Keywords: CDI; Clostridium difficile; Clostridium difficile infection; asymptomatic colonization; microbiota.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

