Brain tumor biobanking in the precision medicine era: building a high-quality resource for translational research in neuro-oncology
- PMID: 29692920
- PMCID: PMC5909804
- DOI: 10.1093/nop/npw029
Brain tumor biobanking in the precision medicine era: building a high-quality resource for translational research in neuro-oncology
Erratum in
-
Corrigendum.Neurooncol Pract. 2017 Jun;4(2):136. doi: 10.1093/nop/npx006. Epub 2017 May 30. Neurooncol Pract. 2017. PMID: 31386007 Free PMC article.
Abstract
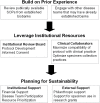
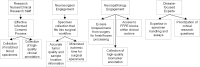
The growth of precision medicine has made access to biobanks with high-quality, well-annotated neuro-oncology biospecimens critical. Developing and maintaining neuro-oncology biobanks is best accomplished through multidisciplinary collaboration between clinicians and researchers. Balancing the needs and leveraging the skills of all stakeholders in this multidisciplinary effort is of utmost importance. Collaboration with a multidisciplinary team of clinicians, health care team members, and institutions, as well as patients and their families, is essential for access to participants in order to obtain informed consent, collect samples under strict standard operating procedures, and accurate and relevant clinical annotation. Once a neuro-oncology biobank is established, development and implementation of policies related to governance and distribution of biospecimens (both within and outside the institution) is of critical importance for sustainability. Proper implementation of a governance process helps to ensure that the biospecimens and data can be utilized in research with the largest potential benefit. New NIH and peer-reviewed journal policies related to public sharing of 'omic' data generated from stored biospecimens create new ethical challenges that must be addressed in developing informed consents, protocols, and standard operating procedures. In addition, diversification of sources of funding for the biobanks is needed for long-term sustainability.
Keywords: biobanking; biospecimens; brain tumors.
Figures
References
-
- National Institutes of Health. NOT-OD-03-032: Final NIH Statement on Sharing Research Data http://grants.nih.gov/grants/guide/notice-files/NOT-OD-03-032.html Published February 26, 2003. Accessed November 3, 2016.
-
- National Institutes of Health. NOT-OD-14-124: NIH Genomic Data Sharing Policy http://grants.nih.gov/grants/guide/notice-files/NOT-OD-14-124.html Published August 27, 2014. Accessed November 3, 2016.
-
- Louis DN, Ohgaki H, Wiestler OD, Cavanee WK, eds. WHO Classification of Tumours of the Central Nervous System. Lyon, France: International Agency for Research on Cancer; 2016.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources