Murine Nephrotoxic Nephritis as a Model of Chronic Kidney Disease
- PMID: 29692933
- PMCID: PMC5859794
- DOI: 10.1155/2018/8424502
Murine Nephrotoxic Nephritis as a Model of Chronic Kidney Disease
Abstract
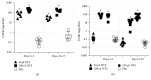
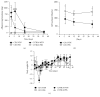
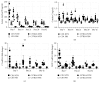
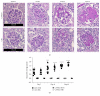
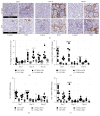
Using the nonaccelerated murine nephrotoxic nephritis (NTN) as a model of chronic kidney disease (CKD) could provide an easily inducible model that enables a rapid test of treatments. Originally, the NTN model was developed as an acute model of glomerulonephritis, but in this study we evaluate the model as a CKD model and compare CD1 and C57BL/6 female and male mice. CD1 mice have previously showed an increased susceptibility to CKD in other CKD models. NTN was induced by injecting nephrotoxic serum (NTS) and evaluated by CKD parameters including albuminuria, glomerular filtration rate (GFR), mesangial expansion, and renal fibrosis. Both strains showed significant albuminuria on days 2-3 which remained significant until the last time point on days 36-37 supporting dysfunctional filtration also observed by a significantly declined GFR on days 5-6, 15-17, and 34-37. Both strains showed early progressive mesangial expansion and significant renal fibrosis within three weeks suggesting CKD development. CD1 and C57BL/6 females showed a similar disease progression, but female mice seemed more susceptible to NTS compared to male mice. The presence of albuminuria, GFR decline, mesangial expansion, and fibrosis showed that the NTN model is a relevant CKD model both in C57BL/6 and in CD1 mice.
Figures



References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

