A simple method to determine evaporation and compensate for liquid losses in small-scale cell culture systems
- PMID: 29693210
- PMCID: PMC5990580
- DOI: 10.1007/s10529-018-2556-x
A simple method to determine evaporation and compensate for liquid losses in small-scale cell culture systems
Abstract
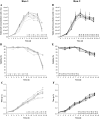
Objectives: Establish a method to indirectly measure evaporation in microwell-based cell culture systems and show that the proposed method allows compensating for liquid losses in fed-batch processes.
Results: A correlation between evaporation and the concentration of Na+ was found (R2 = 0.95) when using the 24-well-based miniature bioreactor system (micro-Matrix) for a batch culture with GS-CHO. Based on these results, a method was developed to counteract evaporation with periodic water additions based on measurements of the Na+ concentration. Implementation of this method resulted in a reduction of the relative liquid loss after 15 days of a fed-batch cultivation from 36.7 ± 6.7% without volume corrections to 6.9 ± 6.5% with volume corrections.
Conclusion: A procedure was established to indirectly measure evaporation through a correlation with the level of Na+ ions in solution and deriving a simple formula to account for liquid losses.
Keywords: Evaporation; Fed batch; GS-CHO cells; Miniature shaken bioreactor; Reproducibility.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



References
-
- Betts JPJ, Warr SRC, Finka GB, et al. Impact of aeration strategies on fed-batch cell culture kinetics in a single-use 24-well miniature bioreactor. Biochem Eng J. 2014;82:105–116. doi: 10.1016/j.bej.2013.11.010. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

