Ellagic Acid Alleviates Hepatic Oxidative Stress and Insulin Resistance in Diabetic Female Rats
- PMID: 29693586
- PMCID: PMC5986411
- DOI: 10.3390/nu10050531
Ellagic Acid Alleviates Hepatic Oxidative Stress and Insulin Resistance in Diabetic Female Rats
Abstract
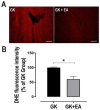
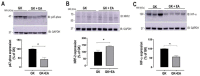
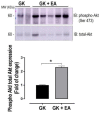
Non-alcoholic fatty liver disease (NAFLD) affects more than 70% of patients with type 2 diabetes mellitus (T2DM) and has become one of the most common metabolic liver diseases worldwide. To date, treatments specifically targeting NAFLD do not exist. Oxidative stress and insulin resistance have been implicated in the pathogenesis of NAFLD in diabetes. Accordingly, the goal of this present study was to determine whether Ellagic acid (EA), a natural antioxidant polyphenol found in berries and nuts, mitigates hepatic oxidative stress and insulin resistance in T2DM rats, and thus alleviates NAFLD. Using adult female Goto Kakizaki (GK) rats, a non-obese and spontaneous model of T2DM, we found that EA treatment significantly lowered fasting blood glucose and reduced insulin resistance, as shown by a 21.8% reduction in the homeostasis model assessment index of insulin resistance (HOMA-IR), while triglyceride and total cholesterol levels remained unchanged. Increased hepatic lipid accumulation and oxidative stress present in diabetic GK rats was markedly reduced with EA treatment. This effect was associated with a downregulation of the NADPH oxidase subunit, p47-phox, and overexpression of NF-E2-related factor-2 (NRF2). Moreover, EA was able to decrease the hepatic expression of hypoxia-inducible factor (HIF-α), a transcription factor linked to hypoxia and hepatic steatosis. We further showed that EA treatment activated an insulin signaling pathway in the liver, as evidenced by increased levels of phosphorylated Akt (Ser 473). In conclusion, our results demonstrate that EA diminishes blood glucose levels and potently suppress NAFLD in diabetic rats via mechanisms that involve reductions in p47-phox and HIF-α, upregulation of NRF2 and enhancement of the Akt signaling pathway in the liver. Together, these results reveal that EA improves hepatic insulin sensitivity and lipid metabolism as a result of its antioxidant effects. This implies an anti-diabetic effect of EA with beneficial effects for the treatment of hepatic complications in T2DM.
Keywords: Ellagic Acid; Goto-kakizaki (GK) rats; HIF-α; NRF2; Type II Diabetes Mellitus (T2DM); antioxidant; hepatic steatosis; insulin resistance; oxidative stress; p47-phox.
Conflict of interest statement
The authors declare no conflict of interest. The funding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures



Similar articles
-
Molecular mechanism of Fufang Zhenzhu Tiaozhi capsule in the treatment of type 2 diabetes mellitus with nonalcoholic fatty liver disease based on network pharmacology and validation in minipigs.J Ethnopharmacol. 2021 Jun 28;274:114056. doi: 10.1016/j.jep.2021.114056. Epub 2021 Mar 23. J Ethnopharmacol. 2021. PMID: 33771638
-
Ellagic acid ameliorates oxidative stress and insulin resistance in high glucose-treated HepG2 cells via miR-223/keap1-Nrf2 pathway.Biomed Pharmacother. 2019 Feb;110:85-94. doi: 10.1016/j.biopha.2018.11.018. Epub 2018 Nov 19. Biomed Pharmacother. 2019. PMID: 30466006
-
Tangganjian decoction ameliorates type 2 diabetes mellitus and nonalcoholic fatty liver disease in rats by activating the IRS/PI3K/AKT signaling pathway.Biomed Pharmacother. 2018 Oct;106:733-737. doi: 10.1016/j.biopha.2018.06.089. Epub 2018 Jul 11. Biomed Pharmacother. 2018. PMID: 29990865
-
The Effect of Low Glycemic Index and Glycemic Load Diets on Hepatic Fat Mass, Insulin Resistance, and Blood Lipid Panels in Individuals with Nonalcoholic Fatty Liver Disease.Metab Syndr Relat Disord. 2019 Oct;17(8):389-396. doi: 10.1089/met.2019.0038. Epub 2019 Jul 15. Metab Syndr Relat Disord. 2019. PMID: 31305201 Review.
-
Improvements in Metabolic Health with Consumption of Ellagic Acid and Subsequent Conversion into Urolithins: Evidence and Mechanisms.Adv Nutr. 2016 Sep 15;7(5):961-72. doi: 10.3945/an.116.012575. Print 2016 Sep. Adv Nutr. 2016. PMID: 27633111 Free PMC article. Review.
Cited by
-
Berberis aristata DC Extract Counteracts the High Fat Diet-Induced Reproductive Toxicity in Female Wistar Rats via Modulating Oxidative Stress and Resistance to Leptin and Insulin.Endocr Metab Immune Disord Drug Targets. 2022;22(14):1390-1402. doi: 10.2174/1871530322666220429125241. Endocr Metab Immune Disord Drug Targets. 2022. PMID: 35579124
-
Pharmacological Properties of Polyphenols: Bioavailability, Mechanisms of Action, and Biological Effects in In Vitro Studies, Animal Models, and Humans.Biomedicines. 2021 Aug 23;9(8):1074. doi: 10.3390/biomedicines9081074. Biomedicines. 2021. PMID: 34440278 Free PMC article. Review.
-
Nanoliposome-encapsulated ellagic acid prevents cyclophosphamide-induced rat liver damage.Mol Cell Biochem. 2019 Aug;458(1-2):185-195. doi: 10.1007/s11010-019-03541-8. Epub 2019 Apr 19. Mol Cell Biochem. 2019. PMID: 31004308
-
Clinically Effective Molecules of Natural Origin for Obesity Prevention or Treatment.Int J Mol Sci. 2024 Feb 25;25(5):2671. doi: 10.3390/ijms25052671. Int J Mol Sci. 2024. PMID: 38473918 Free PMC article. Review.
-
Design and Optimization of a Second-Generation Extruded Snack Using Carrot Waste, Blue Corn Flour, and Ellagic Acid as Functional Ingredients.Foods. 2025 May 8;14(10):1657. doi: 10.3390/foods14101657. Foods. 2025. PMID: 40428437 Free PMC article.
References
-
- Giorda C., Forlani G., Manti R., Mazzella N., De Cosmo S., Rossi M.C., Nicolucci A., Russo G., Di Bartolo P., Ceriello A., et al. Occurrence over time and regression of nonalcoholic fatty liver disease in type 2 diabetes. Diabetes/Metab. Res. Rev. 2017;33:e2878. doi: 10.1002/dmrr.2878. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

