A high-frequency phenotypic switch links bacterial virulence and environmental survival in Acinetobacter baumannii
- PMID: 29693659
- PMCID: PMC5921939
- DOI: 10.1038/s41564-018-0151-5
A high-frequency phenotypic switch links bacterial virulence and environmental survival in Acinetobacter baumannii
Abstract
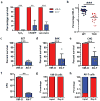
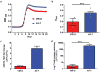
Antibiotic-resistant infections lead to 700,000 deaths per year worldwide 1 . The roles of phenotypically diverse subpopulations of clonal bacteria in the progression of diseases are unclear. We found that the increasingly pathogenic and antibiotic-resistant pathogen Acinetobacter baumannii harbours a highly virulent subpopulation of cells responsible for disease. This virulent subpopulation possesses a thicker capsule and is resistant to host antimicrobials, which drive its enrichment during infection. Importantly, bacteria harvested from the bloodstream of human patients belong exclusively to this virulent subpopulation. Furthermore, the virulent form exhibits increased resistance to hospital disinfectants and desiccation, indicating a role in environmental persistence and the epidemic spread of disease. We identified a transcriptional 'master regulator' of the switch between avirulent and virulent cells, the overexpression of which abrogates virulence. Furthermore, the overexpression strain is capable of vaccinating mice against lethal challenge. This work highlights a phenotypic subpopulation of bacteria that drastically alters the outcome of infection, and illustrates how knowledge of the regulatory mechanisms controlling such phenotypic switches can be harnessed to attenuate bacteria and develop translational interventions.
Conflict of interest statement
Figures


References
-
- O’Neill J. Review on Antimicrobial Resistance: Tackling drug resistant infections globally. London: 2014. https://amr-review.org/sites/default/files/AMR%20Review%20Paper%20-%20Ta....
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

