Smectite for acute infectious diarrhoea in children
- PMID: 29693719
- PMCID: PMC6494641
- DOI: 10.1002/14651858.CD011526.pub2
Smectite for acute infectious diarrhoea in children
Abstract
Background: As mortality secondary to acute infectious diarrhoea has decreased worldwide, the focus shifts to adjuvant therapies to lessen the burden of disease. Smectite, a medicinal clay, could offer a complementary intervention to reduce the duration of diarrhoea.
Objectives: To assess the effects of smectite for treating acute infectious diarrhoea in children.
Search methods: We searched the Cochrane Infectious Diseases Group Specialized Register, the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE (Pubmed), Embase (Ovid), LILACS, reference lists from studies and previous reviews, and conference abstracts, up to 27 June 2017.
Selection criteria: Randomized and quasi-randomized trials comparing smectite to a control group in children aged one month to 18 years old with acute infectious diarrhoea.
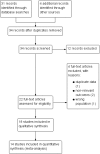
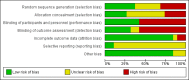
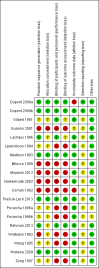
Data collection and analysis: Two review authors independently screened abstracts and the full texts for inclusion, extracted data, and assessed risk of bias. Our primary outcomes were duration of diarrhoea and clinical resolution at day 3. We summarized continuous outcomes using mean differences (MD) and dichotomous outcomes using risk ratios (RR), with 95% confidence intervals (CI). Where appropriate, we pooled data in meta-analyses and assessed heterogeneity. We explored publication bias using a funnel plot.
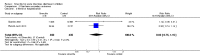
Main results: Eighteen trials with 2616 children met our inclusion criteria. Studies were conducted in both ambulatory and in-hospital settings, and in both high-income and low- or middle-income countries. Most studies included children with rotavirus infections, and half included breastfed children.Smectite may reduce the duration of diarrhoea by approximately a day (MD -24.38 hours, 95% CI -30.91 to -17.85; 14 studies; 2209 children; low-certainty evidence); may increase clinical resolution at day 3 (risk ratio (RR) 2.10, 95% CI 1.30 to 3.39; 5 trials; 312 children; low-certainty evidence); and may reduce stool output (MD -11.37, 95% CI -21.94 to -0.79; 3 studies; 634 children; low-certainty evidence).We are uncertain whether smectite reduces stool frequency, measured as depositions per day (MD -1.33, 95% CI -2.28 to -0.38; 3 studies; 954 children; very low-certainty evidence). There was no evidence of an effect on need for hospitalization (RR 0.93, 95% CI 0.75 to 1.15; 2 studies; 885 children; low-certainty evidence) and need for intravenous rehydration (RR 0.77, 95% CI 0.54 to 1.11; 1 study; 81 children; moderate-certainty evidence). The most frequently reported side effect was constipation, which did not differ between groups (RR 4.71, 95% CI 0.56 to 39.19; 2 studies; 128 children; low-certainty evidence). No deaths or serious adverse effects were reported.
Authors' conclusions: Based on low-certainty evidence, smectite used as an adjuvant to rehydration therapy may reduce the duration of diarrhoea in children with acute infectious diarrhoea by a day; may increase cure rate by day 3; and may reduce stool output, but has no effect on hospitalization rates or need for intravenous therapy.
Conflict of interest statement
Giordano Pérez‐Gaxiola, Carlos A Cuello‐García, Ivan D Florez, Víctor M Pérez‐Pico: we certify that we have no affiliations with or involvement in any organization or entity with a direct financial interest in the subject matter of this Cochrane Review (for example, employment, consultancy, stock ownership, honoraria, or expert testimony).
Figures















Update of
References
References to studies included in this review
-
- Dupont C, Foo JL, Garnier P, Moore N, Mathiex‐Fortunet H, Salazar‐Lindo E, Peru and Malaysia Diosmectite Study Groups. Oral diosmectite reduces stool output and diarrhea duration in children with acute watery diarrhea. Clinical Gastroenterology and Hepatology 2009;7(4):456‐62. - PubMed
-
- Dupont C, Foo JL, Garnier P, Moore N, Mathiex‐Fortunet H, Salazar‐Lindo E, Peru and Malaysia Diosmectite Study Groups. Oral diosmectite reduces stool output and diarrhea duration in children with acute watery diarrhea. Clinical Gastroenterology and Hepatology 2009;7(4):456‐62. - PubMed
-
- Gilbert B, Liendhardt A, Palomera S, Barberis L, Borreda D. The efficacy of smectite in acute infantile diarrhea compared to a placebo and loperamide. Annales de Pediatrie 1991;38(9):633‐6. - PubMed
-
- Guarino A, Bisceglia M, Castellucci G, Iacono G, Casali LG, Bruzzese E, et al. Smectite in the treatment of acute diarrhea: a nationwide randomized controlled study of the Italian Society of Pediatric Gastroenterology and Hepatology (SIGEP) in collaboration with primary care pediatricians. Journal of Pediatric Gastroenterology and Nutrition 2001;32(1):71‐5. - PubMed
-
- Lachaux A, Danzon A, Collet JP, Descos B, Hermier M. Acute infantile diarrhoea. Role of treatment with smectite as complement to rehydration. Randomised double‐blind study. International Review of Pediatrics 1986;163:29‐31.
References to studies excluded from this review
-
- Dupont D, Moreno JL, Barau E, Thiane E, Plique O. Intestinal permeability to lactulose and mannitol during treatment by diosmectite in acute diarrhea in children a double blind vs. placebo study. Gastroenterology 1991;100(5 Pt 2):A684.
-
- Dupont C, Moreno JL, Barau E, Bargaoui K, Thiane E, Plique O. Effect of diosmectite on intestinal permeability changes in acute diarrhea: a double‐blind placebo‐controlled trial. Journal of Pediatric Gastroenterology and Nutrition 1992;14(4):413‐9. - PubMed
-
- Karas J. Smecta pulvis and its place in the treatment of acute rotavirus gastroenteritis of neonates. Československá Pediatrie 1996;51(2):85‐90.
-
- Madkour AA. Placebo‐controlled, double‐blind clinical trial of smectite in acute pediatric diarrhea. Fortschritte der Medizin 1994;112(24):6p. - PubMed
Additional references
-
- Das RR, Sankar J, Naik SS. Efficacy and safety of diosmectite in acute childhood diarrhoea: a meta‐analysis. Archives of Disease in Childhood 2015;100(7):704‐12. - PubMed
-
- McMaster University (developed by Evidence Prime, Inc.). Available from gradepro.org. GRADEpro GDT: GRADEpro Guideline Development Tool. Hamilton, ON: McMaster University (developed by Evidence Prime, Inc.). Available from gradepro.org, 2015.
-
- Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

