Uterotonic agents for preventing postpartum haemorrhage: a network meta-analysis
- PMID: 29693726
- PMCID: PMC6494487
- DOI: 10.1002/14651858.CD011689.pub2
Uterotonic agents for preventing postpartum haemorrhage: a network meta-analysis
Update in
-
Uterotonic agents for preventing postpartum haemorrhage: a network meta-analysis.Cochrane Database Syst Rev. 2018 Dec 19;12(12):CD011689. doi: 10.1002/14651858.CD011689.pub3. Cochrane Database Syst Rev. 2018. Update in: Cochrane Database Syst Rev. 2025 Apr 16;4:CD011689. doi: 10.1002/14651858.CD011689.pub4. PMID: 30569545 Free PMC article. Updated.
Abstract
Background: Postpartum haemorrhage (PPH) is the leading cause of maternal mortality worldwide. Prophylactic uterotonic drugs can prevent PPH, and are routinely recommended. There are several uterotonic drugs for preventing PPH but it is still debatable which drug is best.
Objectives: To identify the most effective uterotonic drug(s) to prevent PPH, and generate a ranking according to their effectiveness and side-effect profile.
Search methods: We searched Cochrane Pregnancy and Childbirth's Trials Register (1 June 2015), ClinicalTrials.gov and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) for unpublished trial reports (30 June 2015) and reference lists of retrieved studies.
Selection criteria: All randomised controlled comparisons or cluster trials of effectiveness or side-effects of uterotonic drugs for preventing PPH.Quasi-randomised trials and cross-over trials are not eligible for inclusion in this review.
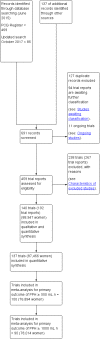
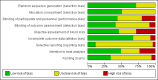
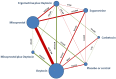
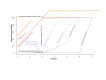
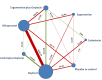
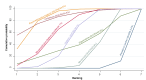
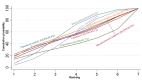
Data collection and analysis: At least three review authors independently assessed trials for inclusion and risk of bias, extracted data and checked them for accuracy. We estimated the relative effects and rankings for preventing PPH ≥ 500 mL and PPH ≥ 1000 mL as primary outcomes. We performed pairwise meta-analyses and network meta-analysis to determine the relative effects and rankings of all available drugs. We stratified our primary outcomes according to mode of birth, prior risk of PPH, healthcare setting, dosage, regimen and route of drug administration, to detect subgroup effects.The absolute risks in the oxytocin are based on meta-analyses of proportions from the studies included in this review and the risks in the intervention groups were based on the assumed risk in the oxytocin group and the relative effects of the interventions.
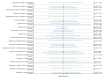
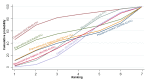
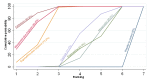
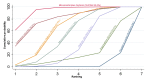
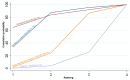
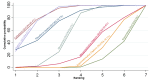
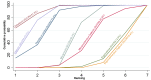
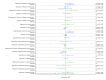
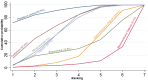
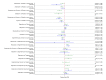
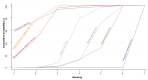
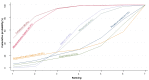
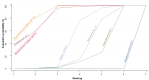
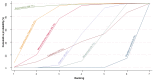
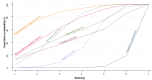
Main results: This network meta-analysis included 140 randomised trials with data from 88,947 women. There are two large ongoing studies. The trials were mostly carried out in hospital settings and recruited women who were predominantly more than 37 weeks of gestation having a vaginal birth. The majority of trials were assessed to have uncertain risk of bias due to poor reporting of study design. This primarily impacted on our confidence in comparisons involving carbetocin trials more than other uterotonics.The three most effective drugs for prevention of PPH ≥ 500 mL were ergometrine plus oxytocin combination, carbetocin, and misoprostol plus oxytocin combination. These three options were more effective at preventing PPH ≥ 500 mL compared with oxytocin, the drug currently recommended by the WHO (ergometrine plus oxytocin risk ratio (RR) 0.69 (95% confidence interval (CI) 0.57 to 0.83), moderate-quality evidence; carbetocin RR 0.72 (95% CI 0.52 to 1.00), very low-quality evidence; misoprostol plus oxytocin RR 0.73 (95% CI 0.60 to 0.90), moderate-quality evidence). Based on these results, about 10.5% women given oxytocin would experience a PPH of ≥ 500 mL compared with 7.2% given ergometrine plus oxytocin combination, 7.6% given carbetocin, and 7.7% given misoprostol plus oxytocin. Oxytocin was ranked fourth with close to 0% cumulative probability of being ranked in the top three for PPH ≥ 500 mL.The outcomes and rankings for the outcome of PPH ≥ 1000 mL were similar to those of PPH ≥ 500 mL. with the evidence for ergometrine plus oxytocin combination being more effective than oxytocin (RR 0.77 (95% CI 0.61 to 0.95), high-quality evidence) being more certain than that for carbetocin (RR 0.70 (95% CI 0.38 to 1.28), low-quality evidence), or misoprostol plus oxytocin combination (RR 0.90 (95% CI 0.72 to 1.14), moderate-quality evidence)There were no meaningful differences between all drugs for maternal deaths or severe morbidity as these outcomes were so rare in the included randomised trials.Two combination regimens had the poorest rankings for side-effects. Specifically, the ergometrine plus oxytocin combination had the higher risk for vomiting (RR 3.10 (95% CI 2.11 to 4.56), high-quality evidence; 1.9% versus 0.6%) and hypertension [RR 1.77 (95% CI 0.55 to 5.66), low-quality evidence; 1.2% versus 0.7%), while the misoprostol plus oxytocin combination had the higher risk for fever (RR 3.18 (95% CI 2.22 to 4.55), moderate-quality evidence; 11.4% versus 3.6%) when compared with oxytocin. Carbetocin had similar risk for side-effects compared with oxytocin although the quality evidence was very low for vomiting and for fever, and was low for hypertension.
Authors' conclusions: Ergometrine plus oxytocin combination, carbetocin, and misoprostol plus oxytocin combination were more effective for preventing PPH ≥ 500 mL than the current standard oxytocin. Ergometrine plus oxytocin combination was more effective for preventing PPH ≥ 1000 mL than oxytocin. Misoprostol plus oxytocin combination evidence is less consistent and may relate to different routes and doses of misoprostol used in the studies. Carbetocin had the most favourable side-effect profile amongst the top three options; however, most carbetocin trials were small and at high risk of bias.Amongst the 11 ongoing studies listed in this review there are two key studies that will inform a future update of this review. The first is a WHO-led multi-centre study comparing the effectiveness of a room temperature stable carbetocin versus oxytocin (administered intramuscularly) for preventing PPH in women having a vaginal birth. The trial includes around 30,000 women from 10 countries. The other is a UK-based trial recruiting more than 6000 women to a three-arm trial comparing carbetocin, oxytocin and ergometrine plus oxytocin combination. Both trials are expected to report in 2018.Consultation with our consumer group demonstrated the need for more research into PPH outcomes identified as priorities for women and their families, such as women's views regarding the drugs used, clinical signs of excessive blood loss, neonatal unit admissions and breastfeeding at discharge. To date, trials have rarely investigated these outcomes. Consumers also considered the side-effects of uterotonic drugs to be important but these were often not reported. A forthcoming set of core outcomes relating to PPH will identify outcomes to prioritise in trial reporting and will inform futures updates of this review. We urge all trialists to consider measuring these outcomes for each drug in all future randomised trials. Lastly, future evidence synthesis research could compare the effects of different dosages and routes of administration for the most effective drugs.
Conflict of interest statement
Ioannis D Gallos (IDG): is a co‐applicant to the UK National Institute for Health Research HTA Project Award 14/139/17 entitled “Uterotonic drugs for preventing postpartum haemorrhage: a network meta‐analysis and cost‐effectiveness analysis”. He has been involved in one or more previous or ongoing trials related to the use of uterotonics for the prevention of PPH that could be eligible for inclusion in this review. He will not participate in any decisions regarding these trials (i.e. assessment for inclusion/exclusion, trial quality, data extraction) for the purposes of this review or future updates – these tasks will be carried out by other members of the team who are not directly involved in the trials. Ferring Pharmaceuticals and Novartis have supplied carbetocin and oxytocin for studies and an ongoing study is supported by WHO/Merck for Mothers. IDG has been supported by the MSD for mothers initiative for travel to a meeting for the study.
Helen M Williams (HMW): is part‐funded by the Birmingham Women’s NHS Foundation Trust, and a co‐applicant to the UK National Institute for Health Research HTA Project Award 14/139/17 entitled “Uterotonic drugs for preventing postpartum haemorrhage: a network meta‐analysis and cost‐effectiveness analysis”. Her salary is part‐funded by Tommy's. She is a member of the Executive Board of Ammalife (UK registered charity 1120236). She has also assisted the administration of activities at a single study site in contribution to a multinational randomised controlled trial of carbetocin versus oxytocin,that could potentially be eligible for inclusion in this review. The trial is sponsored by the World Health Organization and supported by Merck for Mothers. She will not participate in decisions regarding the inclusion of this trial in the review or any tasks related to it such as data extraction or quality assessment.
Malcolm J Price (MP) is funded as a research fellow by the UK Medical Research Council (MRC) Project Award MR/J013595/1, and a co‐applicant to the UK National Institute for Health Research HTA Project Award 14/139/17 entitled “Uterotonic drugs for preventing postpartum haemorrhage: a network meta‐analysis and cost‐effectiveness analysis”.
Aurelio Tobias: none known.
Abi Merriel (AM): was part‐funded by Ammalife (UK Registered Charity 1120236) and the Birmingham Women’s NHS Foundation Trust.
Harold Gee (HG): is a Trustee of Ammalife (UK Registered Charity 1120236).
David Lissauer (DL): was previously a Trustee of Ammalife (UK Registered Charity 1120236).
Vidhya Moorthy: none known.
Mariana Widmer (MW): is involved in an ongoing trial related to the use of uterotonics for the prevention of PPH that could be eligible for inclusion in this review. Ferring Pharmaceuticals and Novartis have supplied carbetocin and oxytocin for the trial and the study is supported by WHO/Merck for Mothers. MW will not participate in any decisions regarding this trial (i.e. assessment for inclusion/exclusion, trial quality, data extraction)for the purposes of this review or future updates – these tasks will be carried out by other members of the team who are not directly involved in the trial.
Özge Tunçalp (OT): is a co‐applicant to the UK National Institute for Health Research HTA Project Award 14/139/17 entitled “Uterotonic drugs for preventing postpartum haemorrhage: a network meta‐analysis and cost‐effectiveness analysis”.
A Metin Gulmezoglu (AMG): is a co‐applicant to the UK National Institute for Health Research HTA Project Award 14/139/17 entitled “Uterotonic drugs for preventing postpartum haemorrhage: a network meta‐analysis and cost‐effectiveness analysis”. AMG was involved in the large multicentre trial (as part of the central coordination unit) which may be included in the review. AMG is involved in an ongoing trial related to the use of uterotonics for the prevention of PPH that could be eligible for inclusion in this review. Ferring Pharmaceuticals and Novartis have supplied carbetocin and oxytocin for the trial and the study is supported by WHO/Merck for Mothers. AMG will not participate in any decisions regarding this or previous trials (i.e. assessment for inclusion/exclusion, trial quality, data extraction)for the purposes of this review or future updates – these tasks will be carried out by other members of the team who are not directly involved in the trial.
Jonathan J Deeks (JJD): is a co‐applicant to the UK National Institute for Health Research HTA Project Award 14/139/17 entitled “Uterotonic drugs for preventing postpartum haemorrhage: a network meta‐analysis and cost‐effectiveness analysis”.
G Justus Hofmeyr (GJH) has been and continues to be involved in a number of studies that may be eligible for inclusion in this review, but will not participate in data extraction or quality assessment of the studies in which he was involved. He is a co‐investigator on the UK National Institute for Health Research HTA Project Award 14/139/17 entitled "Uterotonic drugs for preventing postpartum haemorrhage: a network meta‐analysis and cost‐effectiveness analysis". Neither he nor his institution receives funding from this grant.
Arri Coomarasamy (AC): is the Chief Investigator of UK National Institute for Health Research HTA Project Award 14/139/17 entitled "Uterotonic drugs for preventing postpartum haemorrhage: a network meta‐analysis and cost‐effectiveness analysis". He has been involved in one or more previous or ongoing trials related to the use of uterotonics for the prevention of PPH that could be eligible for inclusion in this review. Ferring Pharmaceuticals and Novartis have supplied carbetocin and oxytocin for studies and an ongoing study is supported by WHO/Merck for Mothers. AC will not participate in any decisions regarding these trials (i.e. assessment for inclusion/exclusion, trial quality, data extraction)for the purposes of this review or future updates – these tasks will be carried out by other members of the team who are not directly involved in the trials. AC is a member of the Executive Board of Ammalife (UK registered charity 1120236). He does not receive any payment for this relationship.
Figures





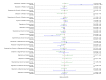
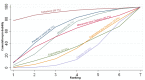

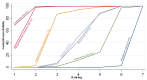

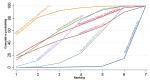


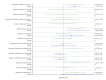
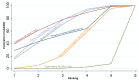
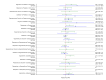
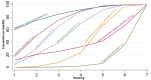





References
References to studies included in this review
-
- Abdel‐Aleem H, Singata M, Abdel‐Aleem M, Mshweshwe N, Williams X, Hofmeyr GJ. Uterine massage to reduce postpartum hemorrhage after vaginal delivery. International Journal of Gynecology & Obstetrics 2010;111(1):32‐6. - PubMed
-
- Acharya G, Al‐Sammarai MT, Patel N, Al‐Habib A, Kiserud T. A randomized, controlled trial comparing effect of oral misoprostol and intravenous syntocinon on intra‐operative blood loss during cesarean section. Acta Obstetricia et Gynecologica Scandinavica 2001;80(3):245‐50. - PubMed
-
- Adanikin AI, Orji EO, Adanikin PO, Olaniyan O. Comparative study of rectal misoprostol to oxytocin infusion in preventing postpartum haemorrhage post‐caesarean section. International Journal of Gynecology & Obstetrics 2012;119(Suppl 3):S825.
- Adanikin AI, Orji EO, Fasubaa OB, Onwudiegwu U, Ijarotimi OA, Olaniyan O. The effect of post‐cesarean rectal misoprostol on intestinal motility. International Journal of Gynecology and Obstetrics 2012;119(2):159‐62. - PubMed
- Orji EO, Adanikin AI. Prospective randomised double blind study on the effect of post‐caesarean rectal misoprostol on intestinal motility. International Journal of Gynecology and Obstetrics 2012;119(Suppl 3):S446‐S447. - PubMed
- Orji EO, Adanikin AO. The effect of post‐caesarean rectal misoprostol on intestinal motility. BJOG: an international journal of obstetrics and gynaecology 2013;120(Suppl s1):21. - PubMed
-
- Afolabi EO, Kuti O, Orji EO, Ogunniyi SO. Oral misoprostol versus intramuscular oxytocin in the active management of the third stage of labour. Singapore Medical Journal 2010;51(3):207‐11. - PubMed
-
- Ahmed WAS, Ibrahim ZM, Mostafa I, Kishk EA, Elbahie MA. Safety and efficacy of carbetocin in hypertensive pregnant women undergoing cesarean delivery. Journal of Maternal‐Fetal & Neonatal Medicine 2014;27(Suppl 1):49.
References to studies excluded from this review
-
- Abdel‐Aleem H, Abol‐Oyoun EM, Moustafa SAM, Kamel HS, Abdel‐Wahab HA. Carboprost trometamol in the management of the third stage of labor. International Journal of Gynecology & Obstetrics 1993;42:247‐50. - PubMed
-
- Abdel‐Aleem H. Management of the third stage of labour with carboprost trometamol in high risk patients for postpartum haemorrhage. Personal communication1997.
- Abdel‐Aleem H, Mostafa SAM, Makarem MH, Abol‐Oyoun EM, Makhlouf A, Shoukry M. Management of the third stage of labour with carboprost trometamol in high risk patients for postpartum hemorrhage. Research activities on reproductive health: annual report of Assiut University Department of Obstetrics and Gynecology November 1997. Assiut University, Faculty of Medicine, 1997:75.
-
- Abdel‐Aleem H, Alhusaini TK, Abdel‐Aleem MA, Menoufy M, Gulmezoglu AM. Effectiveness of tranexamic acid on blood loss in patients undergoing elective cesarean section: randomized clinical trial. Journal of Maternal‐Fetal & Neonatal Medicine 2013;26(17):1705‐9. - PubMed
-
- Abdollahy F. Comparison effect of oxytocin and normal salin injection intra umbelical venuse [abstract]. Gynecological Endocrinology 2000;14(Suppl 2):49.
-
- Al‐Harazi AH, Frass KA. Sublingual misoprostol for the prevention of postpartum hemorrhage. Saudi Medical Journal 2009;30(7):912‐6. - PubMed
References to studies awaiting assessment
-
- Adanikin AI, Orji E, Adanikin PO, Olaniyan O. Comparative study of rectal misoprostol to oxytocin infusion in preventing postpartum haemorrhage after caesarean section. Nepal Journal of Obstetrics and Gynaecology 2013;8(2):34‐7.
-
- Adhikari S, Rana A, Bista KD. Active management of third stage of labour: comparison between prophylactic intramuscular methylergometrine and intramuscular oxytocin. Nepal Journal of Obstetrics and Gynaecology 2007;2(2):24‐8.
-
- Ahmed MR, Sayed Ahmed WA, Madny EH, Arafa AM, Said MM. Efficacy of tranexamic acid in decreasing blood loss in elective caesarean delivery. Journal of Maternal‐Fetal & Neonatal Medicine 2015;28(9):1014‐8. - PubMed
-
- Akinaga C, Uchizaki S, Kurita T, Taniguchi M, Makino H, Suzuki A, et al. Randomized double‐blind comparison of the effects of intramyometrial and intravenous oxytocin during elective cesarean section. Journal of Obstetrics and Gynaecology Research 2016;42(4):404‐9. - PubMed
-
- Ali R, Hina F. Postpartum hemorrhage; comparison of efficacy of ergometrine with misoprostol in prophylaxis in cesarean section. Professional Medical Journal 2012;19(3):360‐4.
References to ongoing studies
-
- NCT01733329. Buccal misoprostol during cesarean section for preventing postpartum hemorrhage in women with risk factors for uterine atony. clinicaltrials.gov/ct2/show/NCT01733329 16 November 2012.
-
- NCT01487278. Comparing misoprostol and oxytocin in UnijectTM postpartum hemorrhage (PPH) prevention in Mali. clinicaltrials.gov/ct2/show/NCT01487278 Date first received: 5 December 2011.
-
- NCT01713153. Comparing misoprostol and oxytocin in uniject for postpartum hemorrhage (PPH) prevention in Senegal. clinicaltrials.gov/ct2/show/NCT01713153 Date first received: 22 October 2012.
-
- Draycott T, Nelson HA. Intramuscular oxytocics: a comparison study of intramuscular carbetocin, syntocinon and syntometrine for the third stage of labour following vaginal birth (IMox). clinicaltrials.gov/ct2/show/NCT02216383 (first received 15 August 2014).
-
- Gomez MCG. Comparison of the effectiveness of carbetocin vs oxytocin in managing the third stage of labor in a group of women with risk factors for postpartum hemorrhage. Australian New Zealand Clinical Trials Registry (www.anzctr.org.au) (accessed 16 February 2011). ACTRN12610000550000 2011.
Additional references
-
- Alkema L, Chou D, Hogan D, Zhang S, Moller AB, Gemmill A, et al. Global, regional, and national levels and trends in maternal mortality between 1990 and 2015, with scenario‐based projections to 2030: a systematic analysis by the UN Maternal Mortality Estimation Inter‐Agency Group. Lancet 2016;387:462‐74. - PMC - PubMed
-
- Combs CA, Murphy EL, Laros RK Jr. Factors associated with postpartum hemorrhage with vaginal birth. Obstetrics and Gynecology 1991;77:69‐76. - PubMed
-
- Davies NM, Longstreth J, Jamali F. Misoprostol therapeutics revisited. Pharmacotherapy 2001;21:60‐73. - PubMed
References to other published versions of this review
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

