Haptoglobin and hemopexin inhibit vaso-occlusion and inflammation in murine sickle cell disease: Role of heme oxygenase-1 induction
- PMID: 29694434
- PMCID: PMC5919001
- DOI: 10.1371/journal.pone.0196455
Haptoglobin and hemopexin inhibit vaso-occlusion and inflammation in murine sickle cell disease: Role of heme oxygenase-1 induction
Abstract
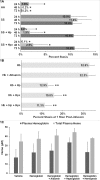
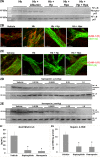
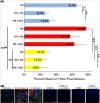
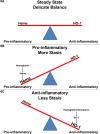
During hemolysis, hemoglobin and heme released from red blood cells promote oxidative stress, inflammation and thrombosis. Plasma haptoglobin and hemopexin scavenge free hemoglobin and heme, respectively, but can be depleted in hemolytic states. Haptoglobin and hemopexin supplementation protect tissues, including the vasculature, liver and kidneys. It is widely assumed that these protective effects are due primarily to hemoglobin and heme clearance from the vasculature. However, this simple assumption does not account for the consequent cytoprotective adaptation seen in cells and organs. To further address the mechanism, we used a hyperhemolytic murine model (Townes-SS) of sickle cell disease to examine cellular responses to haptoglobin and hemopexin supplementation. A single infusion of haptoglobin or hemopexin (± equimolar hemoglobin) in SS-mice increased heme oxygenase-1 (HO-1) in the liver, kidney and skin several fold within 1 hour and decreased nuclear NF-ĸB phospho-p65, and vaso-occlusion for 48 hours after infusion. Plasma hemoglobin and heme levels were not significantly changed 1 hour after infusion of haptoglobin or hemopexin. Haptoglobin and hemopexin also inhibited hypoxia/reoxygenation and lipopolysaccharide-induced vaso-occlusion in SS-mice. Inhibition of HO-1 activity with tin protoporphyrin blocked the protections afforded by haptoglobin and hemopexin in SS-mice. The HO-1 reaction product carbon monoxide, fully restored the protection, in part by inhibiting Weibel-Palade body mobilization of P-selectin and von Willebrand factor to endothelial cell surfaces. Thus, the mechanism by which haptoglobin and hemopexin supplementation in hyperhemolytic SS-mice induces cytoprotective cellular responses is linked to increased HO-1 activity.
Conflict of interest statement
Figures


References
-
- Reiter CD, Wang X, Tanus-Santos JE, Hogg N, Cannon RO 3rd, Schechter AN, et al. Cell-free hemoglobin limits nitric oxide bioavailability in sickle-cell disease. Nat Med. 2002;8(12):1383–9. Epub 2002/11/12. doi: 10.1038/nm799 . - DOI - PubMed
-
- Cooper CE, Silaghi-Dumitrescu R, Rukengwa M, Alayash AI, Buehler PW. Peroxidase activity of hemoglobin towards ascorbate and urate: a synergistic protective strategy against toxicity of Hemoglobin-Based Oxygen Carriers (HBOC). Biochim Biophys Acta. 2008;1784(10):1415–20. Epub 2008/05/07. doi: 10.1016/j.bbapap.2008.03.019 . - DOI - PubMed
-
- Chintagari NR, Jana S, Alayash AI. Oxidized Ferric and Ferryl Forms of Hemoglobin Trigger Mitochondrial Dysfunction and Injury in Alveolar Type I Cells. Am J Respir Cell Mol Biol. 2016;55(2):288–98. Epub 2016/03/15. doi: 10.1165/rcmb.2015-0197OC ; PubMed Central PMCID: PMCPMC4979363. - DOI - PMC - PubMed
-
- Bunn HF, Jandl JH. Exchange of heme among hemoglobins and between hemoglobin and albumin. J Biol Chem. 1968;243(3):465–75. Epub 1968/02/10. . - PubMed
-
- Belcher JD, Chen C, Nguyen J, Milbauer L, Abdulla F, Alayash AI, et al. Heme triggers TLR4 signaling leading to endothelial cell activation and vaso-occlusion in murine sickle cell disease. Blood. 2014;123(3):377–90. doi: 10.1182/blood-2013-04-495887 . - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

