Impairment of chondrocyte proliferation after exposure of young murine cartilage to an aged systemic environment in a heterochronic parabiosis model
- PMID: 29694646
- PMCID: PMC6100763
- DOI: 10.4414/smw.2018.14607
Impairment of chondrocyte proliferation after exposure of young murine cartilage to an aged systemic environment in a heterochronic parabiosis model
Abstract
Aim: The aim of this study was to investigate whether an aged systemic environment could impair young cartilage tissue in mice.
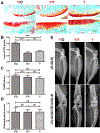
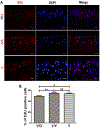
Methods: Mice differing in age were randomly divided into three groups. Group 1 was the experimental group (Y/O group) consisting of the heterochronic parabiosis model (2-month-old/12-month-old, young/old). Group 2 was the surgical control group (Y/Y group) with the isochronic parabiosis model (2-month-old/2-month-old, young/young). Group 3 consisted of the ageing control mice (2-month-old alone, Y group). Young knee cartilages collected from all three groups at 4 months after surgery were compared. Fluorescence molecular tomography (FMT) was used to confirm whether the two mice in parabiosis shared a common blood circulation at 2 weeks after surgery. The knee joints of young mice were examined radiologically at 4 months after surgery. Histological scoring was assigned to grade the severity of osteoarthritis (OA). Immunohistochemistry and quantitative reverse transcription polymerase chain reaction were used to evaluate OA-related protein expression and gene expression, and chondrocyte proliferation was determined with EdU staining.
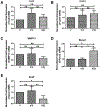
Results: FMT imaging confirmed cross-circulation in the parabiotic pairs. The percentage of EdU-positive chondrocytes in young mice from the Y/O group was significantly lower compared with those of the Y/Y and Y groups (p <0.05 for both). There was no statistically significant difference in the mRNA expression of collagen type II (Col2), collagen type X (Col10), and matrix metalloproteinase 13 (MMP13) among the three groups (P>0.05), but expression of sex-determining region Y box 9 (Sox9) mRNA in young cartilage from the Y/O group was markedly attenuated compared to those in the Y/Y and Y groups (p <0.05 for both). In the Y/O group, mRNA expression of runt-related transcription factor 2 (Runx2) in young cartilage was significantly increased compared to the Y/Y and Y groups (p <0.05 for both). The changes in Col2, Col10, MMP13, Runx2 and Sox9 at the protein level mimicked the alterations found at the mRNA level. Loss of cartilage proteoglycan in young mice from the Y/O group was significantly greater compared to the Y/Y and Y groups (p <0.05 for both), despite the lack of significant difference among the three groups in OARIS and osteophytosis scores.
Conclusion: Heterochronic parabiosis exerts a negative effect on chondrocyte proliferation in the knee cartilage of young mice.
Conflict of interest statement
Potential competing interests
The authors declare that no competing interest exists.
Figures
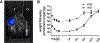

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

