Cytoskeleton Remodeling Induces Membrane Stiffness and Stability Changes of Maturing Reticulocytes
- PMID: 29694877
- PMCID: PMC5937146
- DOI: 10.1016/j.bpj.2018.03.004
Cytoskeleton Remodeling Induces Membrane Stiffness and Stability Changes of Maturing Reticulocytes
Abstract
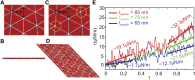
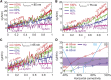
Reticulocytes, the precursors of erythrocytes, undergo drastic alterations in cell size, shape, and deformability during maturation. Experimental evidence suggests that young reticulocytes are stiffer and less stable than their mature counterparts; however, the underlying mechanism is yet to be fully understood. Here, we develop a coarse-grained molecular-dynamics reticulocyte membrane model to elucidate how the membrane structure of reticulocytes contributes to their particular biomechanical properties and pathogenesis in blood diseases. First, we show that the extended cytoskeleton in the reticulocyte membrane is responsible for its increased shear modulus. Subsequently, we quantify the effect of weakened cytoskeleton on the stiffness and stability of reticulocytes, via which we demonstrate that the extended cytoskeleton along with reduced cytoskeleton connectivity leads to the seeming paradox that reticulocytes are stiffer and less stable than the mature erythrocytes. Our simulation results also suggest that membrane budding and the consequent vesiculation of reticulocytes can occur independently of the endocytosis-exocytosis pathway, and thus, it may serve as an additional means of removing unwanted membrane proteins from reticulocytes. Finally, we find that membrane budding is exacerbated when the cohesion between the lipid bilayer and the cytoskeleton is compromised, which is in accord with the clinical observations that erythrocytes start shedding membrane surface at the reticulocyte stage in hereditary spherocytosis. Taken together, our results quantify the stiffness and stability change of reticulocytes during their maturation and provide, to our knowledge, new insights into the pathogenesis of hereditary spherocytosis and malaria.
Copyright © 2018 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures




References
-
- An X., Mohandas N. Erythroblastic islands, terminal erythroid differentiation and reticulocyte maturation. Int. J. Hematol. 2011;93:139–143. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

