Cell Autonomous and Non-cell Autonomous Regulation of SMC Progenitors in Pulmonary Hypertension
- PMID: 29694892
- PMCID: PMC5959296
- DOI: 10.1016/j.celrep.2018.03.043
Cell Autonomous and Non-cell Autonomous Regulation of SMC Progenitors in Pulmonary Hypertension
Abstract
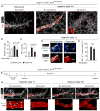
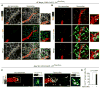
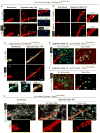
Pulmonary hypertension is a devastating disease characterized by excessive vascular muscularization. We previously demonstrated primed platelet-derived growth factor receptor β+ (PDGFR-β+)/smooth muscle cell (SMC) marker+ progenitors at the muscular-unmuscular arteriole border in the normal lung, and in hypoxia-induced pulmonary hypertension, a single primed cell migrates distally and expands clonally, giving rise to most of the pathological smooth muscle coating of small arterioles. Little is known regarding the molecular mechanisms underlying this process. Herein, we show that primed cell expression of Kruppel-like factor 4 and hypoxia-inducible factor 1-α (HIF1-α) are required, respectively, for distal migration and smooth muscle expansion in a sequential manner. In addition, the HIF1-α/PDGF-B axis in endothelial cells non-cell autonomously regulates primed cell induction, proliferation, and differentiation. Finally, myeloid cells transdifferentiate into or fuse with distal arteriole SMCs during hypoxia, and Pdgfb deletion in myeloid cells attenuates pathological muscularization. Thus, primed cell autonomous and non-cell autonomous pathways are attractive therapeutic targets for pulmonary hypertension.
Keywords: cardiovascular disease; endothelial-smooth muscle cell interactions; pulmonary artery; pulmonary hypertension; pulmonary vascular disease; smooth muscle biology; smooth muscle progenitors; vascular biology; vascular wall; vasculoproliferative disease.
Copyright © 2018 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Amsellem V, Abid S, Poupel L, Parpaleix A, Rodero M, Gary-Bobo G, Latiri M, Dubois-Rande JL, Lipskaia L, Combadiere C, Adnot S. Roles for the CX3CL1/CX3CR1 and CCL2/CCR2 chemokine systems in hypoxic pulmonary hypertension. Am J Respir Cell Mol Biol. 2017;56:597–608. - PubMed
-
- Arias-Stella J, Saldana M. The terminal portion of the pulmonary arterial tree in people native to high altitudes. Circulation. 1963;28:915–925. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

