Rapid Neuromodulation of Layer 1 Interneurons in Human Neocortex
- PMID: 29694902
- PMCID: PMC5946807
- DOI: 10.1016/j.celrep.2018.03.111
Rapid Neuromodulation of Layer 1 Interneurons in Human Neocortex
Abstract
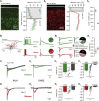
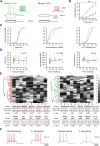
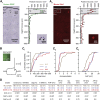
Inhibitory interneurons govern virtually all computations in neocortical circuits and are in turn controlled by neuromodulation. While a detailed understanding of the distinct marker expression, physiology, and neuromodulator responses of different interneuron types exists for rodents and recent studies have highlighted the role of specific interneurons in converting rapid neuromodulatory signals into altered sensory processing during locomotion, attention, and associative learning, it remains little understood whether similar mechanisms exist in human neocortex. Here, we use whole-cell recordings combined with agonist application, transgenic mouse lines, in situ hybridization, and unbiased clustering to directly determine these features in human layer 1 interneurons (L1-INs). Our results indicate pronounced nicotinic recruitment of all L1-INs, whereas only a small subset co-expresses the ionotropic HTR3 receptor. In addition to human specializations, we observe two comparable physiologically and genetically distinct L1-IN types in both species, together indicating conserved rapid neuromodulation of human neocortical circuits through layer 1.
Keywords: cell types; evolution; genetic markers; human neocortex; interneuron types; layer 1 interneurons; mouse neocortex; neocortical circuits; neuromodulation; translation; whole-cell recordings.
Copyright © 2018 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

