Dimerization of the voltage-sensing phosphatase controls its voltage-sensing and catalytic activity
- PMID: 29695412
- PMCID: PMC5940254
- DOI: 10.1085/jgp.201812064
Dimerization of the voltage-sensing phosphatase controls its voltage-sensing and catalytic activity
Abstract
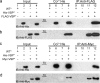
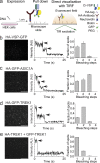
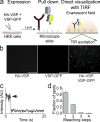
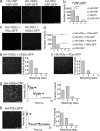
Multimerization is a key characteristic of most voltage-sensing proteins. The main exception was thought to be the Ciona intestinalis voltage-sensing phosphatase (Ci-VSP). In this study, we show that multimerization is also critical for Ci-VSP function. Using coimmunoprecipitation and single-molecule pull-down, we find that Ci-VSP stoichiometry is flexible. It exists as both monomers and dimers, with dimers favored at higher concentrations. We show strong dimerization via the voltage-sensing domain (VSD) and weak dimerization via the phosphatase domain. Using voltage-clamp fluorometry, we also find that VSDs cooperate to lower the voltage dependence of activation, thus favoring the activation of Ci-VSP. Finally, using activity assays, we find that dimerization alters Ci-VSP substrate specificity such that only dimeric Ci-VSP is able to dephosphorylate the 3-phosphate from PI(3,4,5)P3 or PI(3,4)P2 Our results indicate that dimerization plays a significant role in Ci-VSP function.
© 2018 Rayaprolu et al.
Figures

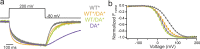

References
-
- Calebiro D., Rieken F., Wagner J., Sungkaworn T., Zabel U., Borzi A., Cocucci E., Zürn A., and Lohse M.J.. 2013. Single-molecule analysis of fluorescently labeled G-protein-coupled receptors reveals complexes with distinct dynamics and organization. Proc. Natl. Acad. Sci. USA. 110:743–748. 10.1073/pnas.1205798110 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

