Targeting protein biotinylation enhances tuberculosis chemotherapy
- PMID: 29695454
- PMCID: PMC6151865
- DOI: 10.1126/scitranslmed.aal1803
Targeting protein biotinylation enhances tuberculosis chemotherapy
Abstract
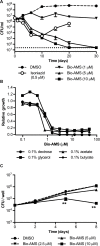
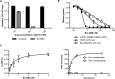
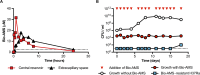
Successful drug treatment for tuberculosis (TB) depends on the unique contributions of its component drugs. Drug resistance poses a threat to the efficacy of individual drugs and the regimens to which they contribute. Biologically and chemically validated targets capable of replacing individual components of current TB chemotherapy are a major unmet need in TB drug development. We demonstrate that chemical inhibition of the bacterial biotin protein ligase (BPL) with the inhibitor Bio-AMS (5'-[N-(d-biotinoyl)sulfamoyl]amino-5'-deoxyadenosine) killed Mycobacterium tuberculosis (Mtb), the bacterial pathogen causing TB. We also show that genetic silencing of BPL eliminated the pathogen efficiently from mice during acute and chronic infection with Mtb Partial chemical inactivation of BPL increased the potency of two first-line drugs, rifampicin and ethambutol, and genetic interference with protein biotinylation accelerated clearance of Mtb from mouse lungs and spleens by rifampicin. These studies validate BPL as a potential drug target that could serve as an alternate frontline target in the development of new drugs against Mtb.
Copyright © 2018 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Figures



References
-
- W. H. Organization , Antimicrobial Resistance: Global Report on Surveillance (World Health Organization, 2014).
-
- Draper P., The outer parts of the mycobacterial envelope as permeability barriers. Front. Biosci.3: D1253–D1261 (1998). - PubMed
-
- Nguyen L., Pieters J., Mycobacterial subversion of chemotherapeutic reagents and host defense tactics: Challenges in tuberculosis drug development. Annu. Rev. Pharmacol. Toxicol. 49, 427–453 (2009). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

