Insights into the binding behavior of native and non-native cytochromes to photosystem I from Thermosynechococcus elongatus
- PMID: 29695502
- PMCID: PMC5995507
- DOI: 10.1074/jbc.RA117.000953
Insights into the binding behavior of native and non-native cytochromes to photosystem I from Thermosynechococcus elongatus
Abstract
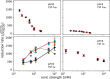
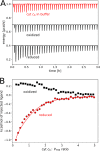
The binding of photosystem I (PS I) from Thermosynechococcus elongatus to the native cytochrome (cyt) c6 and cyt c from horse heart (cyt cHH) was analyzed by oxygen consumption measurements, isothermal titration calorimetry (ITC), and rigid body docking combined with electrostatic computations of binding energies. Although PS I has a higher affinity for cyt cHH than for cyt c6, the influence of ionic strength and pH on binding is different in the two cases. ITC and theoretical computations revealed the existence of unspecific binding sites for cyt cHH besides one specific binding site close to P700 Binding to PS I was found to be the same for reduced and oxidized cyt cHH Based on this information, suitable conditions for cocrystallization of cyt cHH with PS I were found, resulting in crystals with a PS I:cyt cHH ratio of 1:1. A crystal structure at 3.4-Å resolution was obtained, but cyt cHH cannot be identified in the electron density map because of unspecific binding sites and/or high flexibility at the specific binding site. Modeling the binding of cyt c6 to PS I revealed a specific binding site where the distance and orientation of cyt c6 relative to P700 are comparable with cyt c2 from purple bacteria relative to P870 This work provides new insights into the binding modes of different cytochromes to PS I, thus facilitating steps toward solving the PS I-cyt c costructure and a more detailed understanding of natural electron transport processes.
Keywords: complex; crystallography; cytochrome c; docking; photosynthesis; photosystem I.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures




References
-
- Stieger K. R., Feifel S. C., Lokstein H., Hejazi M., Zouni A., and Lisdat F. (2016) Biohybrid architectures for efficient light-to-current conversion based on photosystem I within scalable 3D mesoporous electrodes. J. Mater. Chem. A 4, 17009–17017 10.1039/C6TA07141D - DOI
-
- Stieger K. R., Ciornii D., Kölsch A., Hejazi M., Lokstein H., Feifel S. C., Zouni A., and Lisdat F. (2016) Engineering of supramolecular photoactive protein architectures: the defined co-assembly of photosystem I and cytochrome c using a nanoscaled DNA-matrix. Nanoscale 8, 10695–10705 10.1039/C6NR00097E - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

