Novel role of prostate apoptosis response-4 tumor suppressor in B-cell chronic lymphocytic leukemia
- PMID: 29695515
- PMCID: PMC6024641
- DOI: 10.1182/blood-2017-10-813931
Novel role of prostate apoptosis response-4 tumor suppressor in B-cell chronic lymphocytic leukemia
Abstract
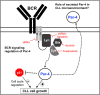
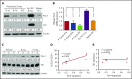
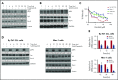
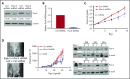
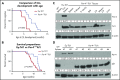
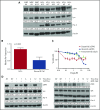
Prostate apoptosis response-4 (Par-4), a proapoptotic tumor suppressor protein, is downregulated in many cancers including renal cell carcinoma, glioblastoma, endometrial, and breast cancer. Par-4 induces apoptosis selectively in various types of cancer cells but not normal cells. We found that chronic lymphocytic leukemia (CLL) cells from human patients and from Eµ-Tcl1 mice constitutively express Par-4 in greater amounts than normal B-1 or B-2 cells. Interestingly, knockdown of Par-4 in human CLL-derived Mec-1 cells results in a robust increase in p21/WAF1 expression and decreased growth due to delayed G1-to-S cell-cycle transition. Lack of Par-4 also increased the expression of p21 and delayed CLL growth in Eμ-Tcl1 mice. Par-4 expression in CLL cells required constitutively active B-cell receptor (BCR) signaling, as inhibition of BCR signaling with US Food and Drug Administration (FDA)-approved drugs caused a decrease in Par-4 messenger RNA and protein, and an increase in apoptosis. In particular, activities of Lyn, a Src family kinase, spleen tyrosine kinase, and Bruton tyrosine kinase are required for Par-4 expression in CLL cells, suggesting a novel regulation of Par-4 through BCR signaling. Together, these results suggest that Par-4 may play a novel progrowth rather than proapoptotic role in CLL and could be targeted to enhance the therapeutic effects of BCR-signaling inhibitors.
© 2018 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures


References
-
- Dighiero G, Hamblin TJ. Chronic lymphocytic leukaemia. Lancet. 2008;371(9617):1017-1029. - PubMed
-
- Chiorazzi N, Rai KR, Ferrarini M. Chronic lymphocytic leukemia. N Engl J Med. 2005;352(8):804-815. - PubMed
-
- Gururajan M, Jennings CD, Bondada S. Cutting edge: constitutive B cell receptor signaling is critical for basal growth of B lymphoma. J Immunol. 2006;176(10):5715-5719. - PubMed
-
- Jain N, O’Brien S. Targeted therapies for CLL: practical issues with the changing treatment paradigm. Blood Rev. 2016;30(3):233-244. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

