ABBY: A phase 2 randomized trial of crenezumab in mild to moderate Alzheimer disease
- PMID: 29695589
- PMCID: PMC5962917
- DOI: 10.1212/WNL.0000000000005550
ABBY: A phase 2 randomized trial of crenezumab in mild to moderate Alzheimer disease
Abstract
Objective: To evaluate the safety and efficacy of crenezumab in patients with mild to moderate Alzheimer disease (AD).
Methods: In this phase 2 trial, 431 patients with mild to moderate AD 50 to 80 years of age were randomized 2:1 (crenezumab:placebo). Patients received low-dose subcutaneous crenezumab 300 mg or placebo every 2 weeks (n = 184) or high-dose intravenous crenezumab 15 mg/kg or placebo every 4 weeks (n = 247) for 68 weeks. Primary outcome measures were change in Alzheimer's Disease Assessment Scale-Cognitive Subscale (ADAS-Cog12) and Clinical Dementia Rating-Sum of Boxes scores from baseline to week 73.
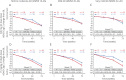
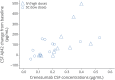
Results: The primary and secondary endpoints were not met. In an exploratory post hoc analysis, a reduction in decline on the ADAS-Cog12 was observed in the high-dose group. Separation from the placebo group on the ADAS-Cog12 was greatest in the milder subsets of AD patients and reached statistical significance in the group with Mini-Mental State Examination scores of 22 to 26. In both groups, there was a significant increase in CSF β-amyloid1-42 levels that correlated with crenezumab CSF levels. The overall rate of adverse events was balanced between groups. One case of amyloid-related imaging abnormalities indicative of vasogenic edema or effusions was reported.
Conclusions: Although prespecified criteria for testing treatment effects were not met, these data suggest a potential treatment effect in patients with mild AD treated with high-dose crenezumab. Together with the safety profile for crenezumab, these data support the exploration of crenezumab treatment at even higher doses in patients with early AD.
Clinicaltrialsgov identifier: NCT 01343966.
Classification of evidence: This study provides Class II evidence that, for people with AD, crenezumab does not significantly improve cognition or function at 18 months. The study is rated Class II because <80% of enrolled patients completed the study.
© 2018 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology.
Figures
References
-
- Alzheimer's Association Report. Alzheimer's disease facts and figures. Alzheimers Dement 2017;13:325–373.
-
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science 2002;297:353–356. - PubMed
-
- Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer's amyloid beta-peptide. Nat Rev Mol Cell Biol 2007;8:101–112. - PubMed
-
- Benilova I, Karran E, De Strooper B. The toxic Aβ oligomer and Alzheimer's disease: an emperor in need of clothes. Nat Neurosci 2012;15:349. - PubMed
-
- Wang ZX, Tan L, Liu J, Yu JT. The essential role of soluble Aβ oligomers in Alzheimer's disease. Mol Neurobiol 2016;53:1905. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
