Cryo-EM structure of substrate-bound human telomerase holoenzyme
- PMID: 29695869
- PMCID: PMC6223129
- DOI: 10.1038/s41586-018-0062-x
Cryo-EM structure of substrate-bound human telomerase holoenzyme
Abstract
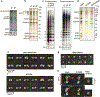
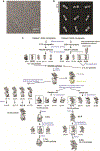
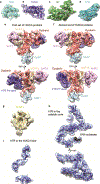
The enzyme telomerase adds telomeric repeats to chromosome ends to balance the loss of telomeres during genome replication. Telomerase regulation has been implicated in cancer, other human diseases, and ageing, but progress towards clinical manipulation of telomerase has been hampered by the lack of structural data. Here we present the cryo-electron microscopy structure of the substrate-bound human telomerase holoenzyme at subnanometre resolution, showing two flexibly RNA-tethered lobes: the catalytic core with telomerase reverse transcriptase (TERT) and conserved motifs of telomerase RNA (hTR), and an H/ACA ribonucleoprotein (RNP). In the catalytic core, RNA encircles TERT, adopting a well-ordered tertiary structure with surprisingly limited protein-RNA interactions. The H/ACA RNP lobe comprises two sets of heterotetrameric H/ACA proteins and one Cajal body protein, TCAB1, representing a pioneering structure of a large eukaryotic family of ribosome and spliceosome biogenesis factors. Our findings provide a structural framework for understanding human telomerase disease mutations and represent an important step towards telomerase-related clinical therapeutics.
Figures





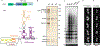
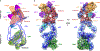
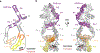
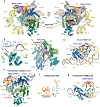
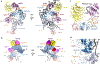
Comment in
-
Detailed view of human telomerase enzyme invites rethink of its structure.Nature. 2018 May;557(7704):174-175. doi: 10.1038/d41586-018-04756-3. Nature. 2018. PMID: 29730671 No abstract available.
References
-
- Levy MZ, Allsopp RC, Futcher AB, Greider CW & Harley CB Telomere end-replication problem and cell aging. J. Mol. Biol. 225, 951–960 (1992). - PubMed
-
- Wegman-Ostrosky T & Savage SA The genomics of inherited bone marrow failure: from mechanism to the clinic. Br. J. Haematol. 177, 526–542 (2017). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

