Interfacing Graphene-Based Materials With Neural Cells
- PMID: 29695956
- PMCID: PMC5904258
- DOI: 10.3389/fnsys.2018.00012
Interfacing Graphene-Based Materials With Neural Cells
Abstract
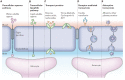
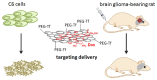
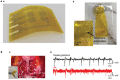
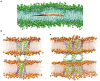
The scientific community has witnessed an exponential increase in the applications of graphene and graphene-based materials in a wide range of fields, from engineering to electronics to biotechnologies and biomedical applications. For what concerns neuroscience, the interest raised by these materials is two-fold. On one side, nanosheets made of graphene or graphene derivatives (graphene oxide, or its reduced form) can be used as carriers for drug delivery. Here, an important aspect is to evaluate their toxicity, which strongly depends on flake composition, chemical functionalization and dimensions. On the other side, graphene can be exploited as a substrate for tissue engineering. In this case, conductivity is probably the most relevant amongst the various properties of the different graphene materials, as it may allow to instruct and interrogate neural networks, as well as to drive neural growth and differentiation, which holds a great potential in regenerative medicine. In this review, we try to give a comprehensive view of the accomplishments and new challenges of the field, as well as which in our view are the most exciting directions to take in the immediate future. These include the need to engineer multifunctional nanoparticles (NPs) able to cross the blood-brain-barrier to reach neural cells, and to achieve on-demand delivery of specific drugs. We describe the state-of-the-art in the use of graphene materials to engineer three-dimensional scaffolds to drive neuronal growth and regeneration in vivo, and the possibility of using graphene as a component of hybrid composites/multi-layer organic electronics devices. Last but not least, we address the need of an accurate theoretical modeling of the interface between graphene and biological material, by modeling the interaction of graphene with proteins and cell membranes at the nanoscale, and describing the physical mechanism(s) of charge transfer by which the various graphene materials can influence the excitability and physiology of neural cells.
Keywords: blood-brain barrier; brain; computational modeling; graphene; nanomedicine; neurology; scaffolds; smart materials.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

