The Effects of PPAR Stimulation on Cardiac Metabolic Pathways in Barth Syndrome Mice
- PMID: 29695963
- PMCID: PMC5904206
- DOI: 10.3389/fphar.2018.00318
The Effects of PPAR Stimulation on Cardiac Metabolic Pathways in Barth Syndrome Mice
Abstract
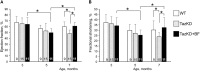
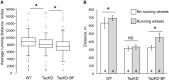
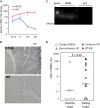
Aim: Tafazzin knockdown (TazKD) in mice is widely used to create an experimental model of Barth syndrome (BTHS) that exhibits dilated cardiomyopathy and impaired exercise capacity. Peroxisome proliferator-activated receptors (PPARs) are a group of nuclear receptor proteins that play essential roles as transcription factors in the regulation of carbohydrate, lipid, and protein metabolism. We hypothesized that the activation of PPAR signaling with PPAR agonist bezafibrate (BF) may ameliorate impaired cardiac and skeletal muscle function in TazKD mice. This study examined the effects of BF on cardiac function, exercise capacity, and metabolic status in the heart of TazKD mice. Additionally, we elucidated the impact of PPAR activation on molecular pathways in TazKD hearts. Methods: BF (0.05% w/w) was given to TazKD mice with rodent chow. Cardiac function in wild type-, TazKD-, and BF-treated TazKD mice was evaluated by echocardiography. Exercise capacity was evaluated by exercising mice on the treadmill until exhaustion. The impact of BF on metabolic pathways was evaluated by analyzing the total transcriptome of the heart by RNA sequencing. Results: The uptake of BF during a 4-month period at a clinically relevant dose effectively protected the cardiac left ventricular systolic function in TazKD mice. BF alone did not improve the exercise capacity however, in combination with everyday voluntary running on the running wheel BF significantly ameliorated the impaired exercise capacity in TazKD mice. Analysis of cardiac transcriptome revealed that BF upregulated PPAR downstream target genes involved in a wide spectrum of metabolic (energy and protein) pathways as well as chromatin modification and RNA processing. In addition, the Ostn gene, which encodes the metabolic hormone musclin, is highly induced in TazKD myocardium and human failing hearts, likely as a compensatory response to diminished bioenergetic homeostasis in cardiomyocytes. Conclusion: The PPAR agonist BF at a clinically relevant dose has the therapeutic potential to attenuate cardiac dysfunction, and possibly exercise intolerance in BTHS. The role of musclin in the failing heart should be further investigated.
Keywords: Barth syndrome; Ostn; PPAR agonist; bezafibrate; cardiomyopathy; musclin; tafazzin.
Figures



References
-
- Barreto-Torres G., Hernandez J. S., Jang S., Rodriguez-Munoz A. R., Torres-Ramos C. A., Basnakian A. G., et al. (2015). The beneficial effects of AMP kinase activation against oxidative stress are associated with prevention of PPARalpha-cyclophilin D interaction in cardiomyocytes. Am. J. Physiol. Heart Circ. Physiol. 308 H749–H758. 10.1152/ajpheart.00414.2014 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

