Adverse Drug Reactions in Hospitalized Patients: Results of the FORWARD (Facilitation of Reporting in Hospital Ward) Study
- PMID: 29695966
- PMCID: PMC5904209
- DOI: 10.3389/fphar.2018.00350
Adverse Drug Reactions in Hospitalized Patients: Results of the FORWARD (Facilitation of Reporting in Hospital Ward) Study
Abstract
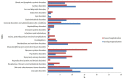
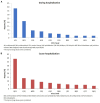
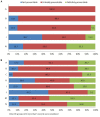
Background: Adverse drug reactions (ADRs) are an important public health problem, representing a major cause of morbidity and mortality. However, several countries have no recent studies available. Since 2014, a prospective active pharmacovigilance project, aimed to improve ADRs monitoring in hospital wards (FORWARD) was performed in Sicily. This study, as part of FORWARD project, was aimed to describe ADRs occurred during the hospital stay in Internal Medicine wards. ADRs related to hospital admission, characteristics and preventability of ADRs were also evaluated. Methods: Demographic, clinical, and pharmacological data on patients admitted to six wards of Internal Medicine, from 2014 to 2015, were collected by trained, qualified monitors, who screened all medical records. The rate of ADRs occurred during hospital stay and those leading to hospitalization were analyzed. A descriptive analysis of the reactions, suspected drugs, and associated factors was performed according to the setting analyzed. Results: During the study period, 4,802 admissions were recorded; in 3.2% of them ADRs occurred during hospital stay while in 6.2%, admission was due to ADRs. The duration of hospital stay was longer in patients who experienced ADRs during hospitalization, compared to patients without ADRs [median days 12 (Q1-Q3: 8-17) vs. 9 (6-13)]; p < 0.001). Females [OR1.39 (95% CI 1.03-1.93)] and patients taking ≥ 4 drugs [OR1.46 (95% CI 1.06-2.03)] were more likely to experience ADRs during hospital stay, as well as to be admitted because of ADRs [female: OR1.75 (95% CI 1.37-2.24); ≥ 4 drugs: OR2.14 (95% CI 1.67-2.74)]. The most frequent ADRs occurred during hospital stay were cutaneous (26.8%), general (13.4%), vascular (13.4%), and cardiac (11.5%) disorders and the drug classes mainly involved were anti-bacterials (38.2%) and antithrombotic agents (21.7%). ADRs were serious in 44.6% and probably preventable in 69.4%. Gastrointestinal (27.7%), hematological (26.5%), metabolic (18.1%), and nervous (16.1%) disorders were the main ADRs cause of hospitalization, primarily due to antithrombotic agents (39.0%) RAS-inhibitors (13.9%), NSAIDs (11.9%), and diuretics (9.0%). Only 12.9% of them was not preventable. Conclusion: Adverse drug reactions occurred during hospitalization or contributing to admission to Internal Medicine wards were considerable and most of them were preventable. Females and patients taking many medications were more likely to present ADRs both during hospital stay or as cause of admission.
Keywords: adverse drug reactions; elderly; hospital admission; internal medicine; pharmacovigilance; preventability; risk factors.
Figures
References
-
- Aranaz-Andres J. M., Aibar-Remon C., Vitaller-Murillo J., Ruiz-Lopez P., Limon-Ramirez R., Terol-Garcia E., et al. (2008). Incidence of adverse events related to health care in Spain: results of the Spanish National Study of Adverse Events. J. Epidemiol. Community Health 62 1022–1029. 10.1136/jech.2007.065227 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

