Aromatic Amino Acid-Derived Compounds Induce Morphological Changes and Modulate the Cell Growth of Wine Yeast Species
- PMID: 29696002
- PMCID: PMC5904269
- DOI: 10.3389/fmicb.2018.00670
Aromatic Amino Acid-Derived Compounds Induce Morphological Changes and Modulate the Cell Growth of Wine Yeast Species
Abstract
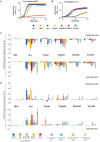
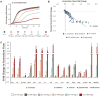
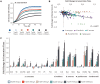
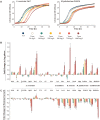
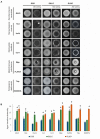
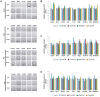
Yeasts secrete a large diversity of compounds during alcoholic fermentation, which affect growth rates and developmental processes, like filamentous growth. Several compounds are produced during aromatic amino acid metabolism, including aromatic alcohols, serotonin, melatonin, and tryptamine. We evaluated the effects of these compounds on growth parameters in 16 different wine yeasts, including non-Saccharomyces wine strains, for which the effects of these compounds have not been well-defined. Serotonin, tryptamine, and tryptophol negatively influenced yeast growth, whereas phenylethanol and tyrosol specifically affected non-Saccharomyces strains. The effects of the aromatic alcohols were observed at concentrations commonly found in wines, suggesting a possible role in microbial interaction during wine fermentation. Additionally, we demonstrated that aromatic alcohols and ethanol are able to affect invasive and pseudohyphal growth in a manner dependent on nutrient availability. Some of these compounds showed strain-specific effects. These findings add to the understanding of the fermentation process and illustrate the diversity of metabolic communication that may occur among related species during metabolic processes.
Keywords: aromatic alcohols; invasive growth; non-Saccharomyces; pseudohyphal growth; quorum sensing; serotonin; tryptamine.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

