Rapid Formation of Microbe-Oil Aggregates and Changes in Community Composition in Coastal Surface Water Following Exposure to Oil and the Dispersant Corexit
- PMID: 29696005
- PMCID: PMC5904270
- DOI: 10.3389/fmicb.2018.00689
Rapid Formation of Microbe-Oil Aggregates and Changes in Community Composition in Coastal Surface Water Following Exposure to Oil and the Dispersant Corexit
Abstract
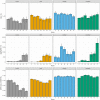
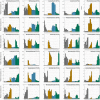
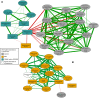
During the Deepwater Horizon (DWH) oil spill, massive quantities of oil were deposited on the seafloor via a large-scale marine oil-snow sedimentation and flocculent accumulation (MOSSFA) event. The role of chemical dispersants (e.g., Corexit) applied during the DWH oil spill clean-up in helping or hindering the formation of this MOSSFA event are not well-understood. Here, we present the first experiment related to the DWH oil spill to specifically investigate the relationship between microbial community structure, oil and Corexit®, and marine oil-snow in coastal surface waters. We observed the formation of micron-scale aggregates of microbial cells around droplets of oil and dispersant and found that their rate of formation was directly related to the concentration of oil within the water column. These micro-aggregates are potentially important precursors to the formation of larger marine oil-snow particles. Therefore, our observation that Corexit® significantly enhanced their formation suggests dispersant application may play a role in the development of MOSSFA events. We also observed that microbial communities in marine surface waters respond to oil and oil plus Corexit® differently and much more rapidly than previously measured, with major shifts in community composition occurring within only a few hours of experiment initiation. In the oil-amended treatments without Corexit®, this manifested as an increase in community diversity due to the outgrowth of several putative aliphatic- and aromatic-hydrocarbon degrading genera, including phytoplankton-associated taxa. In contrast, microbial community diversity was reduced in mesocosms containing chemically dispersed oil. Importantly, different consortia of hydrocarbon degrading bacteria responded to oil and chemically dispersed oil, indicating that functional redundancy in the pre-spill community likely results in hydrocarbon consumption in both undispersed and dispersed oils, but by different bacterial taxa. Taken together, these data improve our understanding of how dispersants influence the degradation and transport of oil in marine surface waters following an oil spill and provide valuable insight into the early response of complex microbial communities to oil exposure.
Keywords: MOSSFA; deepwater horizon; marine oil-snow; micro-aggregate; oil and corexit®.
Figures




References
-
- Alonso-Gutiérrez J., Figueras A., Albaiges J., Jimenez N., Vinas M., Solanas A. M., et al. (2009). Bacterial communities from shoreline environments (costa da morte, northwestern spain) affected by the prestige oil spill. Appl. Environ. Microbiol. 75, 3407–3418. 10.1128/AEM.01776-08 - DOI - PMC - PubMed
-
- Apprill A., McNally S., Parsons R., Weber L. (2015). Minor revision to V4 region SSU rRNA 806R gene primer greatly increases detection of SAR11 bacterioplankton. Aquat. Microb. Ecol. 75, 129–137. 10.3354/ame01753 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

