Soluble N-Ethylmaleimide-Sensitive Factor Attachment Protein Receptor-Derived Peptides for Regulation of Mast Cell Degranulation
- PMID: 29696021
- PMCID: PMC5904360
- DOI: 10.3389/fimmu.2018.00725
Soluble N-Ethylmaleimide-Sensitive Factor Attachment Protein Receptor-Derived Peptides for Regulation of Mast Cell Degranulation
Abstract
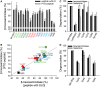
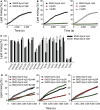
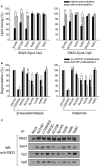
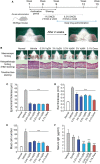
Vesicle-associated V-soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) proteins and target membrane-associated T-SNAREs (syntaxin 4 and SNAP-23) assemble into a core trans-SNARE complex that mediates membrane fusion during mast cell degranulation. This complex plays pivotal roles at various stages of exocytosis from the initial priming step to fusion pore opening and expansion, finally resulting in the release of the vesicle contents. In this study, peptides with the sequences of various SNARE motifs were investigated for their potential inhibitory effects against SNARE complex formation and mast cell degranulation. The peptides with the sequences of the N-terminal regions of vesicle-associated membrane protein 2 (VAMP2) and VAMP8 were found to reduce mast cell degranulation by inhibiting SNARE complex formation. The fusion of protein transduction domains to the N-terminal of each peptide enabled the internalization of the fusion peptides into the cells equally as efficiently as cell permeabilization by streptolysin-O without any loss of their inhibitory activities. Distinct subsets of mast cell granules could be selectively regulated by the N-terminal-mimicking peptides derived from VAMP2 and VAMP8, and they effectively decreased the symptoms of atopic dermatitis in mouse models. These results suggest that the cell membrane fusion machinery may represent a therapeutic target for atopic dermatitis.
Keywords: atopy; degranulation; mast cell; membrane fusion; peptide; soluble N-ethylmaleimide-sensitive factor attachment protein receptor.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

