Investigation of procoagulant activity in extracellular vesicles isolated by differential ultracentrifugation
- PMID: 29696077
- PMCID: PMC5912197
- DOI: 10.1080/20013078.2018.1454777
Investigation of procoagulant activity in extracellular vesicles isolated by differential ultracentrifugation
Abstract
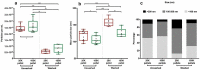
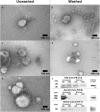
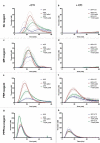
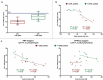
Tissue factor (TF) is the main initiator of coagulation and procoagulant phospholipids (PPL) are key components in promoting coagulation activity in blood. Both TF and PPL may be presented on the surface of extracellular vesicles (EVs), thus contributing to their procoagulant activity. These EVs may constitute a substantial part of pathological hypercoagulability that is responsible for triggering a higher risk of thrombosis in certain patients. The aim of this study was to describe a model system for the isolation of EVs required for investigating their effect on coagulation. Differential ultracentrifugation (DUC) with and without a single washing step was used to isolate and evaluate the procoagulant capacity of EVs from healthy volunteers through analysis of thrombin generation and PPL activity. Ultracentrifugation at 20,000 × g and 100,000 × g resulted in pellets containing larger vesicles and smaller vesicles, respectively. Isolation yield of particle concentration was assessed by nanoparticle tracking analysis. Immunoelectron microscopy and western blotting revealed vesicles positive for the commonly used EV-marker CD9. Plasma proteins and lipoproteins were co-isolated with the EVs; however, application of a washing step clearly diminished the amount of contaminants. The isolated EVs were capable of enhancing thrombin generation, mainly due to PPL predominantly present in pellets from 20,000 × g centrifugation, and correlated with the activity measured by a PPL activity assay. Thus, DUC was proficient for the isolation of EVs with minimal contamination from plasma proteins and lipoproteins, and the setup can be used to study EV-associated procoagulant activity. This may be useful in determining the procoagulant activity of EVs in patients at potentially increased risk of developing thrombosis, e.g. cancer patients. Abbreviations: TF: Tissue factor; PL: Phospholipids; EVs: Extracellular vesicles; FXa: Activated coagulation factor X; TGA: Thrombin generation assay; PPL: Procoagulant phospholipids; DUC: Differential ultracentrifugation; NTA: Nanoparticle tracking analysis; TEM: Transmission electron microscopy; SPP: Standard pool plasma; CTI: Corn trypsin inhibitor; 20K: 20,000 × g; 100K: 100,000 × g; FVIII: Coagulation factor VIII.
Keywords: Electron microscopy; extracellular vesicles; isolation; microvesicles; procoagulant phospholipids; thrombin generation; tissue factor.
Figures

Similar articles
-
Extracellular vesicle-associated procoagulant phospholipid and tissue factor activity in multiple myeloma.PLoS One. 2019 Jan 14;14(1):e0210835. doi: 10.1371/journal.pone.0210835. eCollection 2019. PLoS One. 2019. PMID: 30640949 Free PMC article.
-
Increased activity of procoagulant factors in patients with small cell lung cancer.PLoS One. 2021 Jul 21;16(7):e0253613. doi: 10.1371/journal.pone.0253613. eCollection 2021. PLoS One. 2021. PMID: 34288927 Free PMC article.
-
A sensitive tissue factor activity assay determined by an optimized thrombin generation method.PLoS One. 2023 Jul 19;18(7):e0288918. doi: 10.1371/journal.pone.0288918. eCollection 2023. PLoS One. 2023. PMID: 37467256 Free PMC article.
-
Cancer cell-derived tissue factor-positive extracellular vesicles: biomarkers of thrombosis and survival.Curr Opin Hematol. 2019 Sep;26(5):349-356. doi: 10.1097/MOH.0000000000000521. Curr Opin Hematol. 2019. PMID: 31261175 Free PMC article. Review.
-
Preparation and characterization of extracellular vesicles.Am J Reprod Immunol. 2021 Feb;85(2):e13367. doi: 10.1111/aji.13367. Epub 2020 Nov 16. Am J Reprod Immunol. 2021. PMID: 33118232 Review.
Cited by
-
Expanding applications of allogeneic platelets, platelet lysates, and platelet extracellular vesicles in cell therapy, regenerative medicine, and targeted drug delivery.J Biomed Sci. 2023 Sep 14;30(1):79. doi: 10.1186/s12929-023-00972-w. J Biomed Sci. 2023. PMID: 37704991 Free PMC article. Review.
-
Dynamic cultivation of human mesenchymal stem/stromal cells for the production of extracellular vesicles in a 3D bioreactor system.Biotechnol Lett. 2024 Apr;46(2):279-293. doi: 10.1007/s10529-024-03465-4. Epub 2024 Feb 13. Biotechnol Lett. 2024. PMID: 38349512 Free PMC article.
-
Toward a better understanding of inflammatory microvesicles in alcohol use disorder.J Neurosci Res. 2021 Oct;99(10):2364-2366. doi: 10.1002/jnr.24930. Epub 2021 Jul 22. J Neurosci Res. 2021. PMID: 34292625 Free PMC article. Review. No abstract available.
-
Quantitative Proteomic Analysis of Biogenesis-Based Classification for Extracellular Vesicles.Proteomes. 2020 Nov 6;8(4):33. doi: 10.3390/proteomes8040033. Proteomes. 2020. PMID: 33171920 Free PMC article.
-
Extracellular Vesicles and Their Role in Skin Inflammatory Diseases: From Pathogenesis to Therapy.Int J Mol Sci. 2025 Apr 18;26(8):3827. doi: 10.3390/ijms26083827. Int J Mol Sci. 2025. PMID: 40332512 Free PMC article. Review.
References
-
- Mackman N, Tilley RE, Key NS.. Role of the extrinsic pathway of blood coagulation in hemostasis and thrombosis. Arterioscler Thromb Vasc Biol. 2007;27(8):1687–12. - PubMed
-
- Van Der Pol E, Hoekstra AG, Sturk A, et al. Optical and non-optical methods for detection and characterization of microparticles and exosomes. J Thromb Haemost. 2010;8(12):2596–2607. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

