Conserved Histidine Adjacent to the Proximal Cluster Tunes the Anaerobic Reductive Activation of Escherichia coli Membrane-Bound [NiFe] Hydrogenase-1
- PMID: 29696103
- PMCID: PMC5900901
- DOI: 10.1002/celc.201800047
Conserved Histidine Adjacent to the Proximal Cluster Tunes the Anaerobic Reductive Activation of Escherichia coli Membrane-Bound [NiFe] Hydrogenase-1
Abstract
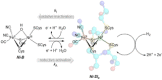
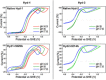
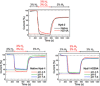
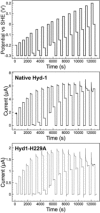
[NiFe] hydrogenases are electrocatalysts that oxidize H2 at a rapid rate without the need for precious metals. All membrane-bound [NiFe] hydrogenases (MBH) possess a histidine residue that points to the electron-transfer iron sulfur cluster closest ("proximal") to the [NiFe] H2-binding active site. Replacement of this amino acid with alanine induces O2 sensitivity, and this has been attributed to the role of the histidine in enabling the reversible O2-induced over-oxidation of the [Fe4S3Cys2] proximal cluster possessed by all O2-tolerant MBH. We have created an Escherichia coli Hyd-1 His-to-Ala variant and report O2-free electrochemical measurements at high potential that indicate the histidine-mediated [Fe4S3Cys2] cluster-opening/closing mechanism also underpins anaerobic reactivation. We validate these experiments by comparing them to the impact of an analogous His-to-Ala replacement in Escherichia coli Hyd-2, a [NiFe]-MBH that contains a [Fe4S4] center.
Keywords: bioinorganic chemistry; electrochemistry; electron transfer; hydrogen; metalloenzymes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Parkin A., in The Metal-Driven Biogeochemistry of Gaseous Compounds in the Environment (Eds.: P. M. H. Kroneck, M. E. S. Torres), Springer Netherlands, Dordrecht, 2014, pp. 99–124.
-
- None
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
