Magnitude and characteristics of clinical trials in the Kingdom of Saudi Arabia: A cross-sectional analysis
- PMID: 29696177
- PMCID: PMC5898478
- DOI: 10.1016/j.conctc.2017.05.008
Magnitude and characteristics of clinical trials in the Kingdom of Saudi Arabia: A cross-sectional analysis
Abstract
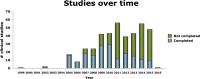
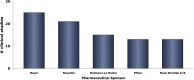
The clinical trial is an important type of research design in the spectrum of translational research. The extent to which clinical trials are conducted is a reflection of the level of advancement that exists within a healthcare system. This study aims at describing the clinical trial activity within the Kingdom of Saudi Arabia since 2000 through reviewing those trials that have been registered with clinicaltrials.gov in that time period. Since February 2000, 405 trials have been registered. These trials fall into one of 22 different ICD-10 codes, and with the top four being neoplasms (92), diseases of the circulatory system (57), endocrine, nutritional and metabolic diseases (46), and diseases of the respiratory system (25). About half (200) were classified as trials with both safety and efficacy endpoints. 52% were phase IV and 28% were phase III. About 64% were randomized, and with about equal numbers of those coming from industry (86) and university sponsors (85), and smaller numbers coming from hospitals (51) and other sponsors. A total of 24 phase III university- or hospital-sponsored trials have been registered during the 15-year time period. With a population approaching 30 million and very large annual healthcare expenses, it would appear that the level of clinical trial activity within the Kingdom during the past 15 years has been rather paltry. The emphasis has been on post-marketing phase IV trials. The academic setting (i.e. universities and hospitals) has seen a new trial registered every 11 months on average.
Figures
References
-
- Simes R.J. Publication bias: the case for an international registry of clinical trials. J. Clin. Oncol. 1986;4(10):1529–1541. - PubMed
-
- Zarin D., Keselman A. Registering a clinical trial in ClinicalTrials.gov. Chest. 2007;131(3):909–912. - PubMed
-
- ClinicalTrialsgov . 2016. Trends, Charts, and Maps.https://clinicaltrials.gov/ct2/resources/trends Available at: (Accessed 18 April 2016)
-
- World Medical Association . 2013. World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects.http://www.wma.net/en/30publications/10policies/b3/index.html Available at: (Accessed 18 April 2016) - PubMed
-
- World Health Organization. International Clinical Trials Registry Platform. Available at: http://www.who.int/ictrp/en/. [Accessed 18 April 2016].
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous