Safety Experience During Real-World Use of Injectable Artesunate in Public Health Facilities in Ghana and Uganda: Outcomes of a Modified Cohort Event Monitoring Study (CEMISA)
- PMID: 29696507
- PMCID: PMC6061362
- DOI: 10.1007/s40264-018-0667-x
Safety Experience During Real-World Use of Injectable Artesunate in Public Health Facilities in Ghana and Uganda: Outcomes of a Modified Cohort Event Monitoring Study (CEMISA)
Abstract
Introduction: Injectable artesunate (Inj AS) is the World Health Organization (WHO)-recommended product for treating severe malaria. However, despite widespread usage, there are few published safety studies involving large populations in real-world settings. In this study, we sought to assess the incidence of common adverse events (AEs) following the intake of Inj AS in real-life settings.
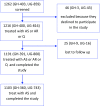
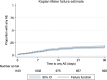
Methods: This is a modified cohort event monitoring study involving patients who were administered with Inj AS at eight sites (four each in Ghana and Uganda) between May and December 2016. Patients were eligible for inclusion if they had severe/complicated malaria and were able and willing to participate in the study. Eligible patients were followed up by telephone or hospital or home visit on Days 7, 14, 21 and 28 after drug administration to document AEs and serious AEs (SAEs). Patients were also encouraged to report all AEs at any time during the study period. The Kaplan-Meier method was used to estimate the proportion of patients with any AEs by end of Day 28. Causality assessment was made on all AEs/SAEs using the WHO/UMC (Uppsala Monitoring Centre) causality method.
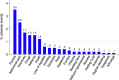
Results: A total of 1103 eligible patients were administered Inj AS, of which 360 patients were in Ghana and 743 in Uganda. The incidence of any AE by the end of follow-up among patients treated with AS was estimated to be 17.9% (197/1103) (95% confidence interval [CI] 15.8-20.3). The median time-to-onset of any AEs was 9 days (interquartile range (IQR) = 4, 14). The top five AEs recorded among patients treated with AS were pyrexia (3.5%), abdominal pain (2.5%), diarrhoea (1.7%), cough (1.5%) and asthenia (1.5%). Most of these top five AEs occurred in the first 14 days following treatment. Regarding the relatedness of these AEs to Inj AS, 78.9% of pyrexia (30/38), 63.0% of pain (17/27), 68.4% of diarrhoea (13/19), 85.5% of cough (14/16) and 75.0% of asthenia (12/16) were assessed as 'possibly' related. There were 17 SAEs including 13 deaths. Two of the deaths are 'possibly' related to Inj AS, as were three non-fatal SAEs: severe abdominal pain, failure of therapy and severe anaemia.
Conclusion: The incidence of common AEs among patients treated with Inj AS in real-world settings was found to be relatively low. Future studies should consider larger cohorts to document rare AEs as well. CLINICALTRIALS.
Gov identifier: NCT02817919.
Conflict of interest statement
Conflicts of interest
H. Hilda Ampadu, Alexander N.O. Dodoo, Samuel Bosomprah, Helga Gardarsdottir, H.G.M. Leufkens, Dan Kajungu and Kwaku Poku Asante have no conflicts of interest. Samantha Akakpo and Pierre Hugo are full-time employees of Medicines for Malaria Venture (MMV).
Funding
Medicines for Malaria Venture provided funding for this study.
Ethical approval
The study received ethical approval from the Ghana Health Service Ethics Review Committee and the Uganda National Council for Science and Technology (UNCST). It was also registered on ClinicalTrials.gov with the ClinicalTrials.gov identifier NCT02817919. The study was conducted under Good Clinical Practice (GCP) guidelines taking into consideration the Declaration of Helsinki (as amended in October 2013) and local rules and regulations of participating countries and health facilities. All personnel involved in the study undertook and successfully passed an online GCP course prior to study initiation unless they already had a valid GCP certificate.
Patient consent
Written informed consent was obtained from the patients for publication of this study. A copy of the written consent may be requested for review from the corresponding author.
Consent for publication
Consent for publication was obtained as part of the informed consent process.
Figures
Similar articles
-
Prescribing patterns and compliance with World Health Organization recommendations for the management of severe malaria: a modified cohort event monitoring study in public health facilities in Ghana and Uganda.Malar J. 2019 Feb 8;18(1):36. doi: 10.1186/s12936-019-2670-9. Malar J. 2019. PMID: 30736864 Free PMC article.
-
Safety Profile of Artemether-Lumefantrine: A Cohort Event Monitoring Study in Public Health Facilities in Tanzania.Clin Drug Investig. 2016 May;36(5):401-11. doi: 10.1007/s40261-016-0385-z. Clin Drug Investig. 2016. PMID: 26951203
-
Safety of a fixed-dose combination of artesunate and amodiaquine for the treatment of uncomplicated Plasmodium falciparum malaria in real-life conditions of use in Côte d'Ivoire.Malar J. 2017 Jan 3;16(1):8. doi: 10.1186/s12936-016-1655-1. Malar J. 2017. PMID: 28049523 Free PMC article.
-
Prospective observational study to evaluate the clinical safety of the fixed-dose artemisinin-based combination Eurartesim® (dihydroartemisinin/piperaquine), in public health facilities in Burkina Faso, Mozambique, Ghana, and Tanzania.Malar J. 2015 Apr 15;14:160. doi: 10.1186/s12936-015-0664-9. Malar J. 2015. PMID: 25885858 Free PMC article.
-
Severe embryotoxicity of artemisinin derivatives in experimental animals, but possibly safe in pregnant women.Molecules. 2009 Dec 25;15(1):40-57. doi: 10.3390/molecules15010040. Molecules. 2009. PMID: 20110870 Free PMC article. Review.
Cited by
-
Spectrophotometric analysis of artesunate injections available in community pharmacies in Northern and Western Uganda.Future Sci OA. 2025 Dec;11(1):2511444. doi: 10.1080/20565623.2025.2511444. Epub 2025 Jun 12. Future Sci OA. 2025. PMID: 40503710 Free PMC article.
-
Quality of care and post-discharge morbidity among children diagnosed with severe malaria in rural Uganda: A prospective cohort study.PLOS Glob Public Health. 2024 Oct 7;4(10):e0003794. doi: 10.1371/journal.pgph.0003794. eCollection 2024. PLOS Glob Public Health. 2024. PMID: 39374246 Free PMC article.
-
Artesunate enhances the efficacy of enzalutamide in advanced prostate cancer.J Biol Chem. 2025 May;301(5):108458. doi: 10.1016/j.jbc.2025.108458. Epub 2025 Mar 26. J Biol Chem. 2025. PMID: 40154619 Free PMC article.
-
Prescribing patterns and compliance with World Health Organization recommendations for the management of severe malaria: a modified cohort event monitoring study in public health facilities in Ghana and Uganda.Malar J. 2019 Feb 8;18(1):36. doi: 10.1186/s12936-019-2670-9. Malar J. 2019. PMID: 30736864 Free PMC article.
References
-
- World Health Organization . World malaria report 2017. Geneva: World Health Organization; 2017. p. 41.
-
- World Health Organization . Guidelines for the treatment of malaria. 3. Geneva: World Health Organization; 2015. - PubMed
-
- Dondorp AM, Fanello CI, Hendriksen IC, Gomes E, Seni A, Chhaganlal KD, et al. Artesunate versus quinine in the treatment of severe falciparum malaria in African children (AQUAMAT): an open-label, randomised trial. Lancet. 2010;376(9753):1647–1657. doi: 10.1016/S0140-6736(10)61924-1. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

