Fostamatinib for the treatment of adult persistent and chronic immune thrombocytopenia: Results of two phase 3, randomized, placebo-controlled trials
- PMID: 29696684
- PMCID: PMC6055608
- DOI: 10.1002/ajh.25125
Fostamatinib for the treatment of adult persistent and chronic immune thrombocytopenia: Results of two phase 3, randomized, placebo-controlled trials
Abstract
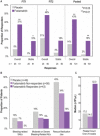
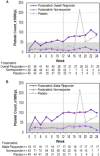
Spleen tyrosine kinase (Syk) signaling is central to phagocytosis-based, antibody-mediated platelet destruction in adults with immune thrombocytopenia (ITP). Fostamatinib, an oral Syk inhibitor, produced sustained on-treatment responses in a phase 2 ITP study. In two parallel, phase 3, multicenter, randomized, double-blind, placebo-controlled trials (FIT1 and FIT2), patients with persistent/chronic ITP were randomized 2:1 to fostamatinib (n = 101) or placebo (n = 49) at 100 mg BID for 24 weeks with a dose increase in nonresponders to 150 mg BID after 4 weeks. The primary endpoint was stable response (platelets ≥50 000/μL at ≥4 of 6 biweekly visits, weeks 14-24, without rescue therapy). Baseline median platelet count was 16 000/μL; median duration of ITP was 8.5 years. Stable responses occurred in 18% of patients on fostamatinib vs. 2% on placebo (P = .0003). Overall responses (defined retrospectively as ≥1 platelet count ≥50 000/μL within the first 12 weeks on treatment) occurred in 43% of patients on fostamatinib vs. 14% on placebo (P = .0006). Median time to response was 15 days (on 100 mg bid), and 83% responded within 8 weeks. The most common adverse events were diarrhea (31% on fostamatinib vs. 15% on placebo), hypertension (28% vs. 13%), nausea (19% vs. 8%), dizziness (11% vs. 8%), and ALT increase (11% vs. 0%). Most events were mild or moderate and resolved spontaneously or with medical management (antihypertensive, anti-motility agents). Fostamatinib produced clinically-meaningful responses in ITP patients including those who failed splenectomy, thrombopoietic agents, and/or rituximab. Fostamatinib is a novel ITP treatment option that targets an important mechanism of ITP pathogenesis.
© 2018 The Authors American Journal of Hematology Published by Wiley Periodicals, Inc.
Figures
References
-
- Rodeghiero F, Stasi R, Gernsheimer T, et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood. 2009;113(11):2386–2393. - PubMed
-
- Cohen YC, Djulbegovic B, Shamai‐Lubovitz O, Mozes B. The bleeding risk and natural history of idiopathic thrombocytopenic purpura in patients with persistent low platelet counts. Arch Intern Med. 2000;160(11):1630–1638. - PubMed
-
- McMillan R, Durette C. Long‐term outcomes in adults with chronic ITP after splenectomy failure. Blood. 2004;104(4):956–960. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
